Overview:
Osimertinib is a third-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) drug. It treats metastatic non-small lung cancer (NSLC), showing positive EGFR expression. Osimertinib, being an EGFR-TKI, is found to be effective in at least 10 % of patients having lung cancer due to the presence of EGFR mutations in the tumor cells. The development of Osimertinib, a third-generation EGFR-TKI, shows alteration in tumor resistance patterns and side effects when administered that impact the quality of life. The use of first-generation EGFR-TKI is found to develop resistance by activating the EGFR gene mutations. And the second-generation EGFR-TKI is proven to be a potent inhibitor. However, the nonspecific targeting of EGFR leads to increased toxicity. But, third-generation drugs like Osimertinib are specific to certain mutations, thereby reducing non-specific binding and minimizing the chances of toxicity.
How Does Osimertinib Work?
Osimertinib is a tyrosine kinase inhibitor that works by blocking the activity of the epidermal growth factor receptor (EGFR), which controls the growth and division of the tumor cells. In lung cancer, the EGFR remains overactive, leading to uncontrolled multiplication of the tumor cells. Thus Osimertinib helps prevent tumor cell growth and division by blocking the EGFR.
Uses:
Osimertinib is indicated for the following.
-
Adjuvant therapy for individuals with non-small cell lung cancer and positive for epidermal growth factor receptor (EGFR) mutation.
-
The first line of treatment for individuals with metastatic non-small cell lung cancer and EGFR mutation.
-
Treatment of individuals with metastatic non-small cell lung cancer and T790M EGFR mutation.
Dosage:
-
Adjuvant Treatment of Early Stage NSLC: Osimertinib can be administered 80 mg orally once daily until disease recurrence and unacceptable toxicity or for up to 3 years. It can be taken with or without food.
-
Treatment of Metastatic NSLC: Osimertinib can be administered 80 mg orally once daily, with or without food, until disease progression and unacceptable toxicity.
Warnings:
-
Pneumonitis or Interstitial Lung Disease: Osimertinib is permanently discontinued if the patient is diagnosed with pneumonitis.
-
QTc Interval Prolongation: Individuals taking medicines that can prolong QTc interval or have risk factors predisposing to prolongation of QTc interval have to be monitored through electrocardiograms and electrolyte levels. In such a case, the drug is withheld and restarted in small doses or permanently discontinued.
-
Cardiomyopathy: Patients with cardiac risks should be monitored before starting the drug dose.
-
Keratitis: An individual with signs and symptoms of keratitis should be evaluated by an ophthalmologist.
-
Erythema Multiforme and Steven-Johnson Syndrome: The drug is permanently discontinued if the patient is suspected of having erythema multiforme and Steven-Johnson syndrome.
-
Cutaneous Vasculitis: Osimertinib is withheld in patients suspected of having cutaneous vasculitis. They are assessed for systemic involvement, and a dermatological consultation is considered. If no other causes are identified, the drug should be discontinued, depending upon the severity of the condition.
-
Embryo-Fetal Toxicity: Males and females are advised to use contraceptives during treatment with Osimertinib as they can be detrimental to the fetus.
For Patients:-
What Do You Need to Know About Lung Cancer?
Lungs are the respiratory organs that inhale oxygen and exhale carbon dioxide. Therefore, individuals who smoke are at a greater risk of lung cancer. Lung cancer is found to be the leading cause of cancer deaths worldwide. Lung cancer occurs not only in individuals who smoke but also in those who have never smoked. Secondhand smoke can also be a reason. The signs and symptoms of lung cancer include shortness of breath, chest pain, coughing blood, losing weight, headache, and bone pain. There are two types of lung cancer, namely, small cell lung cancer and non-small cell lung cancer.
Learn More About Osimertinib:-
Before Starting Osimertinib: Before taking any medication (in this case, Osimertinib), it is good to understand its risks and benefits and discuss it with your doctor to gain knowledge. You may develop severe blistering, peeling of the skin, and ring-like lesions, which must be informed to the physician immediately. This is very important as different people have different medical conditions, and there may be severe reactions if the person is allergic to them.
When and How Often to Take Osimertinib?
The dose of Osimertinib may vary depending on the patient. The following information is an average dose of the drug. For lung cancer, an adult can take 80 milligrams once daily. The dose for children must be decided only by the physician. In addition, the duration of intake depends on the progression of the condition.
How Effective Is Osimertinib?
The effectiveness of Osimertinib has been established through studies where the drug was found to show an effective response in about 62 % of the patients.
Things to Inform Your Doctor Before They Prescribe You Osimertinib:
The following information has to be informed to the physician before they prescribe Osimertinib, which includes whether you:
-
Have respiratory problems.
-
Have heart problems.
-
Have a history of vision problems.
-
Have problems with electrolytes, such as calcium, sodium, potassium, and magnesium.
-
Are pregnant or planning to become pregnant.
-
Are breastfeeding or planning to breastfeed.
-
Are under medications or supplements for any health problems.
Starting Osimertinib:-
How to Take Osimertinib?
-
Osimertinib has to be taken per the healthcare provider's instructions.
-
The drug dose can be altered only by the physician and not by yourself.
-
Osimertinib is recommended to be taken once daily.
-
Osimertinib can be taken with or without food.
-
If a dose is missed, do not take an additional dose to compensate for the missed dose. Instead, follow the next dose regularly according to the schedule.
-
If you cannot swallow the tablet, place the drug in 60 ml of water, stir, and drink the mixture. The tablet should not be broken into pieces as they do not dissolve completely. And once you drink the mixture, pour 120 to 240 ml of water into the container and drink to ensure that you take the complete drug dosage.
Things to Do After You Start Taking Osimertinib:
After you start taking Osimertinib, it is good to schedule regular appointments with your doctor to monitor any adverse effects developed due to the medication. The doctor may conduct a physical and diagnostic test to detect any abnormalities. If any adverse effects are evident, the doctor will decide on the continuation of the drug. Regular appointments can also help monitor the progress of the condition and alter the dose accordingly.
Look Out for Side Effects:
The drug may cause specific side effects that must be monitored and reported to the physician immediately.
The side effects include:
-
Skin rash.
-
Dryness of the skin.
-
Swelling and inflammation of the mouth.
-
Decreased appetite.
-
Constipation.
-
Anxiety.
-
Dizziness.
-
Vision changes.
-
Uneven heartbeat.
-
Chest pain.
-
Pain, redness, and swelling in the arms and legs.
-
Slurred speech.
-
Difficulty breathing.
-
Seizures.
-
Fever.
-
Chills.
-
Blistering and peeling of the skin.
-
Joint and muscle pain.
-
Sore throat.
-
Diarrhea.
-
Ulcers on the mouth and lips.
Dietary Alterations:
Certain drugs may cause adverse reactions when taken along with a particular type of food. So it is good to get the physician’s advice before you start taking Osimertinib. Avoid taking the herb called St.John’s wart along with Osimertinib, as it induces the drug’s metabolism, which makes dose adjustments necessary.
What Should Be Done When You Miss a Dose?
When you miss a dose of Osimertinib, take the next dose according to the schedule and do not overdose to compensate for the missed dose. Any changes in the dose must be made only by the physician and not by yourself.
What Should Be Done to Treat Osimertinib Overdose?
There is no specific antidote for Osimertinib overdose. If you have taken an extra dose of the drug, report immediately to the healthcare provider to monitor and treat the adverse symptoms.
How to Store Osimertinib?
The medication must be stored in its original container at a room temperature between 20 to 25- degree Celsius. The medicines that are out of date must be discarded. This medication must be kept out of the reach of children and pets.
How to Handle Osimertinib?
Keep the medicine in a closed glass container. Try not to touch the tablet with bare hands and wear gloves before handling the drug, as it can cause skin problems. In addition, use a clean container when you mix the tablet with water.
How to Dispose of Osimertinib?
The drug should be discarded by consulting your pharmacist or local waste disposal company but not be flushed in the toilet or thrown in the dustbin.
Avoid Self-Medication:
The tablet should not be taken for a condition that was not prescribed. And do not suggest the tablet to anyone with the same symptoms as you because the medication may show different effects in different individuals. Consult the physician, discuss with them, and then take medicine.
Staying On Osimertinib:-
Tips to Stay On Track: To stay on track with the medicine, take it as directed by the physician. Do not take an overdose or miss a dose. If you do so, inform the physician immediately. Schedule appointments with the physician at regular intervals to check for the disease progression and dose alteration.
For Doctors:-
Indication: Osimertinib is indicated as adjuvant therapy for individuals with non-small cell lung cancer and is positive for epidermal growth factor receptor (EGFR) mutation, the first line of treatment for individuals with metastatic non-small cell lung cancer and EGFR mutation, and treatment of individuals with metastatic non-small cell lung cancer and T790M EGFR mutation.
Pharmacology:
Mechanism of Action:
Osimertinib is a third-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) drug that binds to the EGFR predominant in non-small cell lung cancer tumors. The drug is specific for gate-keeper T790M mutation, which induces the ATP binding to EGFR. It spares the wild-type EGFR, reducing the non-specific binding and limiting toxicity.
Pharmacodynamics:
Pharmacodynamic analysis suggested a concentration-dependent QTc interval prolongation at a dose of Osimertinib 80 mg.
Cardiac Electrophysiology: The QTc interval is the measurement recorded in the electrocardiogram to assess the electrical activity of the heart. The QTc interval prolongation potential of Osimertinib was studied. For this, the data was collected at a steady state and after administration of Osimertinib 80 mg, which showed a significant concentration-dependent QTc interval prolongation.
Chemical Taxonomy:
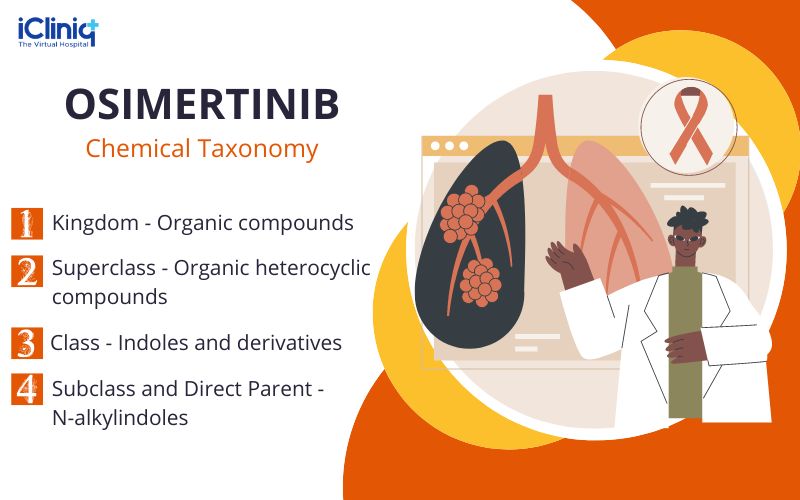
Ingredients:
Active Ingredient: The active ingredient in Osimertinib is Osimertinib.
Inactive Ingredients: The inactive ingredients include mannitol, microcrystalline cellulose, sodium stearyl fumarate, and low-substituted hydroxypropyl cellulose. The tablet coating contains titanium dioxide, polyvinyl alcohol, talc, macrogol 3350, ferric oxide yellow, ferric oxide red, and ferric oxide black.
Absorption:
-
The median time to Cmax (highest concentration of drug in the body after the dose) of Osimertinib is in the range of 3 to 24 hours, on an average of six hours.
-
The administration of 20 mg Osimertinib with a high-fat and high-calorie meal, the Cmax (highest concentration of drug in the body after the dose) and AUC (area under the curve which reflects the actual exposure of the body to the drug after administration) were comparable to the dose under fasting conditions.
Distribution:
-
The mean volume of distribution at a steady state of Osimertinib was 918 L.
-
Plasma protein binding of Osimertinib was 95 %.
Metabolism:
The metabolic pathways of Osimertinib are oxidation and dealkylation in vitro. After oral administration of Osimertinib, active metabolites such as AZ750 and AZ5104 can be identified.
Elimination:
Osimertinib is excreted for about 68 % in feces and 14 % in the urine. 2 % of the elimination accounts for unchanged Osimertinib.
Toxicity:
-
The carcinogenic potential of Osimertinib was tested by oral administration of the drug to rats which showed no carcinogenic response.
-
In vivo and in vitro assays showed no genetic damage caused by Osimertinib.
-
Osimertinib was found to cause male infertility when the rats were administered 80 mg once daily.
-
The administration of Osimertinib in female rats showed no changes in fertility but caused early embryonic deaths.
Warning and Precaution:
-
Pneumonitis or Interstitial Lung Disease: Osimertinib is found to cause pneumonitis in about 3.7 % of 1479 patients included in a study, where 0.3 % were fatal. So Osimertinib must be withheld and tested for pneumonitis in patients with respiratory symptoms such as cough, dyspnea, and fever and is permanently discontinued if the patient is diagnosed with pneumonitis.
-
QTc Interval Prolongation: A study with 1479 patients revealed a QTc interval elongation in patients treated with Osimertinib. Hence individuals taking medicines that can prolong QTc interval or have risk factors predisposing to prolongation of QTc interval have to be monitored through electrocardiograms and electrolyte levels. In such a case, the drug is withheld and restarted in small doses, or the medication is permanently discontinued.
-
Cardiomyopathy: Studies reveal that patients treated with Osimertinib were diagnosed with cardiomyopathy, which was 0.1 % fatal. So patients with cardiac risks should be monitored before starting the drug dose. In case the patient has symptomatic congestive heart failure, Osimertinib has to be discontinued.
-
Keratitis: An individual with signs and symptoms of keratitis, such as eye inflammation, light sensitivity, lacrimation, blurred vision, eye pain, or red eye, should be evaluated by an ophthalmologist before administering Osimertinib.
-
Erythema Multiforme and Steven-Johnson Syndrome: The drug is permanently discontinued if the patient is suspected of having erythema multiforme and Steven-Johnson syndrome.
-
Cutaneous Vasculitis: Studies reveal that Osimertinib can cause cutaneous vasculitis, such as leukocytoclastic vasculitis, urticarial vasculitis, and immunoglobulin A vasculitis. Hence the drug is withheld in patients suspected of having cutaneous vasculitis and is assessed for systemic involvement, and a dermatology consultation is considered. If no other causes are identified, the drug should be discontinued, depending upon the severity of the condition.
-
Embryo-Fetal Toxicity: Osimertinib can cause fetal harm when administered to pregnant female mice. When male mice are treated with the drug before mating with an untreated female, there is an increased chance of preimplantation embryonic loss. And so, the pregnancy status of the female has to be assessed before administering the drug. In addition, males and females are advised to use contraceptives during treatment with Osimertinib
Dosage and Forms:
-
Osimertinib is an orally administered drug.
-
It is available as a tablet.
-
The recommended dosage is 80 mg every 24 hours.
Administration of the Drug:
-
Osimertinib should be taken once daily.
-
The dose of the drug recommended is 80 mg orally.
-
The drug can be taken with or without food.
-
The tablet can be taken as a whole or mixed with water and drunk as a mixture.
-
The dose should be taken regularly and not skipped.
-
If missed, overdose should be avoided.
Considerations for Administration:
-
The co-administration of Osimertinib with strong CYP3A inducers must be avoided as the steady-state AUC of Osimertinib can get reduced by 80 %.
-
The co-administration of Osimertinib with BCRP (breast cancer resistance proteins) substrates must be avoided as they can increase the AUC of BCRP substrates.
-
The administration of drugs in patients with cardiopathy, keratitis, cutaneous vasculitis, erythema multiforme, and Steven-Johnson syndrome should be avoided as they may lead to adverse reactions.
Contraindications:
Osimertinib is contraindicated in the following patients:
-
Hypersensitivity to the ingredients in the drug.
-
History of prolonged QT intervals.
-
History of interstitial lung disease.
-
Erythema multiforme and Steven-Johnson syndrome.
-
Severe renal disorders.
-
Severe hepatic impairment.
-
Pregnant women.
-
Breastfeeding mothers.
Clinical Studies for Osimertinib:
-
The safety of Osimertinib was evaluated in a randomized, double-blind, placebo-controlled trial for the adjuvant treatment of patients with EGFR mutation-positive non-small cell lung cancer who had complete tumor resection, with or without prior chemotherapy. The duration of exposure to the drug was 22.5 months. As a result, serious adverse reactions occurred in 16 % of the patients administered Osimertinib. The adverse reactions leading to dose reduction were diarrhea (4.5 %), stomatitis (3.9 %), rashes (1.8 %), and nail toxicity (1.8 %). The serious adverse effects leading to discontinuation of the drug include interstitial lung disease (2.7 %) and rashes (1.2 %).
-
The safety of Osimertinib was evaluated in a randomized, double-blind, active-controlled trial conducted in 556 patients with EGFR mutation-positive, unresectable or metastatic non-small cell lung cancer who had not received previous systematic treatment. The duration of exposure to the drug was 16.2 months. As a result, serious adverse reactions such as pneumonia, pneumonitis, and pulmonary embolism occurred. The adverse reactions leading to dose reductions were QTc interval prolongation (4.3 %), diarrhea (2.5 %), and lymphopenia (1.1 %). The common adverse effect leading to permanent discontinuation of the drug was pneumonitis.
-
The safety of Osimertinib was evaluated in an open-label randomized controlled trial conducted in 419 patients with EGFR mutation-positive, unresectable or metastatic non-small cell lung cancer who had progressive disease following first-line EGFR TKI treatment. Osimertinib 80 mg orally once daily was administered to 279 patients until tolerance to the therapy or disease progression. A total of 136 patients received Pemetrexed plus Carboplatin or Cisplatin every three weeks for up to six cycles. As a result, serious adverse events occurred in 18 % of patients who received Osimertinib and 26 % of patients who received chemotherapy. No adverse reaction was reported in 2 % of patients on administering Osimertinib, and one patient (0.4 %) treated with Osimertinib experienced a fatal reaction due to pneumonitis. The adverse reactions leading to dose reduction were diarrhea (1.1 %), neutropenia (1.1 %), and QTc interval prolongation (1.8 %). The serious adverse effect leading to permanent discontinuation of the drug was pneumonitis (3 %).
Drug Interactions:
-
Strong CYP3A Inducers: The steady-state AUC of Osimertinib can get reduced by 80 % when administered along with Rifampin, a potent CYP3A inducer.
-
Strong CYP3A Inhibitors: No significant effects were evident on the co-administration of Osimertinib with 200 mg Itraconazole twice daily.
-
Gastric Acid Reducing Agents: The co-administration of Osimertinib and Omeprazole 40 mg for five days showed no significant effects.
-
CYP3A 4 Substrates: The co-administration of Osimertinib and Simvastatin did not show any significant effects.
-
BCRP Substrates: The co-administration of Osimertinib and BCRP (breast cancer resistance proteins) substrates increased the AUC of BCRP substrates.
Other Specifications:-
Osimertinib in Pregnant Women:
There is not enough data on Osimertinib use in pregnant women. But the study of the drug in animals has shown it to harm the fetus. Hence Osimertinib is not recommended during pregnancy.
Osimertinib in Lactating Women:
There is no evidence of the presence of Osimertinib in breast milk. The studies using rats show that the administration of Osimertinib during lactation was associated with adverse effects such as reduced fetal growth rates and neonatal death. Hence, women are advised not to breastfeed during treatment with Osimertinib.
Osimertinib in Pediatric Patients:
The study of Osimertinib in pediatric patients has not yet been established.
Osimertinib in Geriatric Patients:
Studies revealed no significant differences in response to the drug based on age. Hence dose alterations are not required for geriatric patients.
Osimertinib in Renal Impairment Patients:
No dose alterations are recommended in patients with creatinine clearance, and there is no recommended dose for patients with end-stage renal disease.
Osimertinib in Hepatic Impairment Patients:
Osimertinib is not recommended for patients with severe hepatic impairment, and no dose adjustments are required for patients with mild to moderate hepatic impairment.












