Overview:
Belimumab is a recombinant, fully human monoclonal antibody developed by Human Genome Sciences Incorporated and GlaxoSmithKline. It is indicated for treating adult patients with lupus nephritis that occurs in patients with systemic lupus erythematosus (SLE).
Belimumab is directed against the B-cell activating factor (BAFF) or the cytokine BLyS. It is effective in treating adult patients with systemic lupus erythematosus who are already undergoing therapy with a standard treatment regimen.
Belimumab was approved to treat active, autoantibody-positive SLE by the US Food and Drug Administration on March 9, 2011, and by the European Medicines Association (EMA) in July 2011. It is available as an intravenous infusion or subcutaneous injection.
How Does Belimumab Work?
The action of Belimumab is directed against the B-cell activating factor (BAFF) or the cytokine BLyS. When the body is affected by overexpression of BLyS, that can result in B-cell survival, including the autoreactive B-cells. In contrast, inhibition can result in autoreactive B-cell apoptosis (programmed cell death). The elevation of circulating BLyS and subsequent B-cell increase is typical of autoimmune diseases like SLE. Therefore, therapy that reduces or inhibits BLyS activity is considered a useful treatment option. Belimumab is a BLYs-specific inhibitor capable of binding to soluble BLyS and blocking its action - this results in the inhibition of B-cell survival
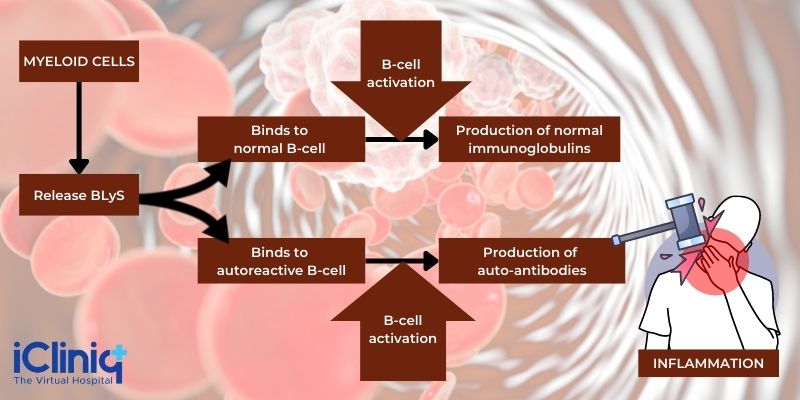

B-Cells: B cells are lymphocytes (a type of white blood cell) responsible for humoral immunity in the body. They produce antibodies as part of their immune response function.
B-Cell Activating Factor (BAFF): Also known as tumor necrosis factor (TNF) ligand superfamily member 13B (TNFSF13B) or B-lymphocyte stimulator (BLyS), BAFF is a necessary factor for the survival and maturation of B cells. BAFF, when overproduced, can result in the occurrence of systemic autoimmune diseases like SLE.
Uses of Belimumab:

- Systemic Lupus Erythematosus: Belimumab is used to treat patients aged five years and above with active, autoantibody-positive SLE undergoing standard therapy.
- Lupus Nephritis: Belimumab is indicated in adult patients with lupus nephritis undergoing standard therapy. However, it has not been evaluated in severe active central nervous system lupus cases and is not indicated in this situation.
Dosage Restrictions:
Routes of Administration:
-
Intravenous infusion.
-
Subcutaneous injection.
Dosage Forms:
-
Intravenous Infusion: Lyophilized powder in single-dose vials (to be reconstituted and diluted before infusion).
-
Subcutaneous Injection: Single-dose prefilled syringe or autoinjector.
Available Strengths:
-
Intravenous Infusion:
-
120 mg lyophilized powder.
-
400 mg lyophilized powder.
-
Subcutaneous Injection:
-
200 mg/ml single-dose prefilled syringe.
-
200 mg/ml single-dose prefilled autoinjector.
Dosage Administration:
- Intravenous Infusion:
Population:
-
Adults with SLE or lupus nephritis.
-
Pediatric patients with SLE.
Dosage:
First 3 doses - 10 mg/kg at two-week intervals (after reconstitution and dilution) - followed by 4-week intervals.
Duration of Administration:
Over an hour.
- Subcutaneous Injection:
Population:
-
Adult patients with SLE or lupus nephritis.
-
Subcutaneous injection is not indicated in children below 18 years of age.
Sites of Injection:
-
Abdomen.
-
Thigh.
Dosage:
-
SLE: 200 mg once weekly.
-
Lupus Nephritis: First 4 doses - 400 mg (two 200 mg injections) once weekly - followed by 200 mg once weekly.
Special Considerations:
- Pregnancy: Pregnant women with SLE may display a variety of adverse reactions, from worsening the disease to spontaneous abortion and premature birth. Belimumab can cross the placenta in the third trimester and lead to negative immune responses in the fetus.
- Lactation: No data has been made available on the presence of Belimumab in human milk or any related concerns such as its effects on infants or on milk production itself.
- Pediatric: Belimumab is contraindicated in children, as its effectiveness and safety in the pediatric population have not been assessed or established.
- Geriatric: Clinical data available on the use of Belimumab in aged patients is insufficient to determine its reliability and safety or reactions. Therefore, it must be used sparingly and with caution.
- Race: The response to Belimumab in black patients has been contradictorily shown to be lower than other races and higher in different phases of trials. This does not provide any conclusive evidence and can only be interpreted to mean that Belimumab must be cautiously administered in black patients.
- Renal Impairment: The use of Belimumab in SLE patients with mild, moderate, or severe renal impairment has been judged to be satisfactory and safe, with no requirements for dosage adjustment.
- Hepatic Impairment: It is not necessary to adjust the Belimumab dosage in SLE patients with hepatic impairment.
- Individuals of Reproductive Age: Females of reproductive age are advised to use contraception during and after administration (for a minimum of four months after the final dose) of Belimumab.
Warnings and Contraindications:
Contraindications: Belimumab is contraindicated in patients who have previously experienced anaphylaxis with the administration of Belimumab.
Warnings and Precautions:
- Hypersensitivity Reactions: Belimumab can elicit severe hypersensitivity reactions, including anaphylaxis. These reactions can lead to death if not immediately managed. Therefore, physicians administering IV Belimumab should do so with extreme caution and be prepared to manage anaphylaxis.
- Infections: Patients undergoing immunosuppressive therapy often display serious infections. Belimumab may also elicit severe infections and are best discontinued in patients who develop infections while undergoing Belimumab therapy. Some of the infections noted with Belimumab are upper respiratory tract infections, urinary infections, and influenza.
- Progressive Multifocal Leukoencephalopathy (PML): PML is associated with the Human polyomavirus 2 and is a rare, fatal viral disease that causes inflammation and damage to the brain’s white matter, resulting in severe neurological deficits. Immunosuppression is a major contributing factor to the development of the disease. SLE patients receiving immunosuppressants, including Belimumab, have been known to develop PML.
- Infusion Reactions: While not typical, infusion reactions have occurred in Belimumab patients. The commonest infusion reactions have included headaches, skin reactions, and nausea.
- Malignancy: Malignancies have developed in a minority of patients who have received Belimumab (both infusions and subcutaneous administration), suggesting that its mechanism of action may contribute to an increased risk of developing malignancy. However, data on this is limited.
- Depression: Patients with a history of depression, other psychiatric conditions, and suicidal ideation may display an increased tendency for depression, anxiety, and suicidal behavior with Belimumab.
- Immunization: Given its mechanism of action, there is a possibility that Belimumab can interfere with vaccination. No data exists on this possibility since it has not been evaluated. It is, however, recommended that vaccines not be administered for 30 days before Belimumab administration or along with Belimumab therapy.
- Mortality: Deaths have been recorded in association with Belimumab administration due to severe infections, cardiovascular disease, and suicide.
- Concomitant Use: Biologic therapies (like B-cell targeted therapy) and intravenous cyclophosphamide are not recommended in combination with Belimumab since these concurrent therapy options have not been evaluated.
For Patients:
What Is Systemic Lupus Erythematosus?
SLE is an autoimmune condition where your immune system attacks tissues and causes destruction and inflammation in your organs (skin, brain, lungs, kidneys, etc.). You can read more on this condition here.
What Is Lupus Nephritis?
Lupus nephritis is a type of kidney disease resulting from SLE, where waste filtration by your kidneys is compromised due to lupus antibodies. This can lead to inflammation of your kidneys along with impaired functioning of the kidneys, high blood pressure, and the presence of blood and protein in the urine. For more information on lupus nephritis, visit this page.
Why Is Belimumab Prescribed for SLE?
Belimumab is an inhibitor of the B-lymphocyte stimulatory cytokine (BLyS), which has been linked to the development and progression of systemic lupus erythematosus. When administered with standard therapy, the efficacy of Belimumab has been demonstrated as superior to that of placebo with standard treatment.
Belimumab is particularly important from a therapeutic perspective due to the multisystem disease activity approach rather than targeting specific organs. This multisystem approach may prevent the all-around escalation and worsening of the disease.
How Should You Take Belimumab?
There are two possible ways to take Belimumab:
-
Intravenous Infusion: Intravenous administration of Belimumab is strictly to be administered by your doctor, who will give you the requisite dose of 10 mg/kg in intervals, and with adequate preparation for circumstances that may arise such as anaphylaxis or other infusion reactions.
-
Subcutaneous Injection: Subcutaneous injections of Belimumab can be self-administered after receiving adequate training from a healthcare professional. The drug is to be injected into either your abdomen or thigh.
How Should You Self-Administer Belimumab?
-
Your doctor or suitable healthcare professional should perform your first SC injection of Belimumab. Following this, you may receive instructions on self-administer Belimumab subcutaneously and are allowed to do so if your doctor deems it appropriate.
-
Remove your autoinjector or syringe from the fridge, and allow it to sit at room temperature 30 minutes before injection. Never attempt to warm the solution in any other way.
-
Visually assess the window of the autoinjector or syringe for discoloration or particulate matter.
-
Do not use the device if you drop it on a hard surface.
-
Your preferred sites for injection should typically be the abdomen or thighs.
-
Never administer the injections in the same area repeatedly. Instead, use different injection sites for each injection.
-
Do not inject into swollen, bruised, tender, red, or hard areas.
-
When administering a 400 mg dose (two 200 mg), ensure the injection sites are spaced at least 5 cm (2 inches) apart.
-
Inject your 200 mg dose once weekly (preferably on the same day each week).
What Should You Discuss With Your Doctor Before Beginning Belimumab Therapy?
- Hypersensitivity Reactions: You should let your doctor know if you have previously received Belimumab therapy and suffered a hypersensitive reaction (including anaphylaxis) in response.
- Pregnancy and Lactation: Belimumab may affect the fetus during a woman's third trimester of pregnancy. Inform your doctor if you are pregnant. The effects of Belimumab on breastfeeding have not been evaluated; however, you will need to keep your doctor posted if you are breastfeeding. This will allow them to form decisions based on your condition.
- Infections: Inform your doctor if you have a history of chronic infections or are currently undergoing treatment for an infection.
- Psychiatric Conditions: If you have been diagnosed with any psychiatric condition (including depression, anxiety, suicidal ideation) or are undergoing treatment for the same, Belimumab may worsen your symptoms. Inform your doctor about your condition and medications before you begin Belimumab therapy.
- Immunization: Notify your doctor if you have received a live vaccine any time between 30 days before you begin Belimumab therapy. Also, ensure you inform them if you are planning to get a live vaccine in the near future so that they may advise you on when you may receive the vaccine instead (since you cannot take the vaccine while being treated with Belimumab).
Is Belimumab Safe?
Data on Belimumab safety has shown that it is well-tolerated with a good safety profile and negligible risk of side effects even on long-term administration.
Is Belimumab Effective?
In combination with standard therapy, Belimumab may increase response rates and prevent the worsening of symptoms of kidney disease in lupus nephritis patients compared to standard treatment alone.
What Side Effects Can You Expect With Belimumab?
-
Infections: The commonest, most severe reaction that can be expected with Belimumab is the occurrence of severe infections such as pneumonia and urinary tract infections.
-
Diarrhea.
-
Pyrexia or Fever.
-
Nasopharyngitis: Inflammation of your pharynx (throat) and nasal cavities.
-
Insomnia: Inability to fall asleep, stay asleep, or get back to sleep after waking up.
-
Bronchitis: Inflammation of the bronchial tube lining. Bronchial tubes are the tubes that carry air to and from the lungs.
-
Migraine.
-
Pain in the Extremities.
-
Depression.
-
Cystitis: Inflammation of the bladder.
-
Pharyngitis: Inflammation of the pharynx or sore throat.
-
Viral Gastroenteritis: Intestinal infection (characterized by inflammation and irritation) caused by viruses such as rotavirus or norovirus.
Can You Stop Treatment With Belimumab Without Your Doctor's Approval?
Belimumab is typically not recommended by doctors if you do not have an active lupus infection or you have not exhausted most other therapeutic options. If your doctor prescribes Belimumab in combination with your standard treatment regimen, you must remember that it is intended as a long-term therapy, and effects may only be shown in 6 months. It may be administered by the doctor as an intravenous infusion, or self-administered via a subcutaneous injection. In either case, never stop treatment unless you are experiencing severe adverse reactions. Whether the therapy does not appear to be effective initially, or notice positive changes, you must continue treatment until your doctor advises otherwise.
What Are the Long-Term Effects of Taking Belimumab?
The long-term effects of Belimumab are pretty positive. However, SLE patients may experience irreversible organ damage that can also contribute to an increased risk of death. Patients who received Belimumab in combination with standard therapy showed less organ damage over five years than patients receiving standard treatment alone or standard treatment with a placebo.
Are There Any Dietary Restrictions to Consider When Taking Belimumab?
No dietary restrictions exist as such with the use of Belimumab. However, you may want to consider an appropriately nutritious diet to aid in controlling symptoms of the disease itself.
How Should You Store and Dispose of Belimumab?
-
Storage: Belimumab must be stored in its original container and kept refrigerated until about 30 minutes before use. If left out for over 12 hours, the medicine may neither be used nor put back into the refrigerator.
-
Disposal: Used needles must be put into a hard, closed container so that needles may not poke through and then disposed of in a place where children and animals may not come in contact with it.
What Can You Do if You Suffer From These Disorders?
Some of the lifestyle modifications you can make when you are suffering from SLE to prevent worsening of symptoms include:
-
Exercise.
-
Stop substance abuse, including smoking, alcohol consumption, and use of recreational or other drugs not recommended by your doctor.
-
Eat a nutritious diet.
What Can You Do in Case of Overdose?
If your healthcare provider administers the dose, you are not likely to experience a Belimumab overdose. However, if you self-administer subcutaneous injections of Belimumab and accidentally overdose, you must seek immediate medical attention. You can call your healthcare provider or local Poison Control Services for more information on managing an overdose. Alternatively, you may seek out emergency medical services.
What Should You Do if You Miss a Dose?
Take your dose as soon as you realize you have missed it. If this is closer to your next dose, then you may proceed with that dose. Do not double-dose yourself under any circumstances.
What Else Should You Keep In Mind?
Keep your doctor informed on your medical history, and ensure you carry information on your condition, medications, or any other conditions you may be suffering from. Contact your doctor if you begin to experience any adverse effects while taking this medication. Hypersensitivity reactions, including itching, rashes, trouble breathing and swallowing, and swelling, must be brought to your doctor's attention.
For Doctors:
Indications:
-
Patients aged five years and above with active, autoantibody-positive systemic lupus erythematosus (SLE) who are receiving standard therapy.
-
Patients aged 18 years and above with active lupus nephritis who are receiving standard therapy.
Limitations:
-
Severe active central nervous system lupus.
-
In combination with other biologics.
Pharmacology:
Description:
-
Type: Human IgG1λ monoclonal antibody.
-
Specific For: BLys (soluble human B lymphocyte stimulator protein).
-
Molecular Weight: 147 kDa.
-
Production: Recombinant DNA technology [murine cell (NS0) expression system].
Intravenous Infusion:
-
Available As: Lyophilized powder (sterile, preservative-free).
-
Color: White to off-white.
-
Packaging: Single-dose vial.
-
Strengths:
-
120 mg per vial.
-
400 mg per vial.
-
Reconstitution: Yes - with sterile water for injection, USP (1.5 mL, 4.8 mL).
-
Concentration to Be Obtained: 80 mg/mL.
-
Withdrawal After Reconstitution:
-
120 mg: 1.5 mL.
-
400 mg: 5 mL.
-
Components per mL:
-
Belimumab 80 mg.
-
Citric acid 0.16 mg.
-
Polysorbate 80 (0.4 mg).
-
Sodium citrate 2.7 mg.
-
Sucrose 80 mg.
-
pH: 6.5.
Subcutaneous Injection:
-
Available As: Solution for subcutaneous injection (sterile, preservative-free).
-
Color: Colorless to pale yellow.
-
Clarity: Clear to opalescent.
-
Packaging or Supply:
Prefilled Autoinjector:
-
1 mL, single-dose.
-
Gauge: 27 gauge.
-
Needle: Half-inch.
-
Components per mL:
-
Belimumab 200 mg.
-
L-arginine hydrochloride 5.3 mg.
-
L-histidine 0.65 mg.
-
L-histidine monohydrochloride 1.2 mg.
-
Polysorbate 80 (0.1 mg).
-
Sodium chloride 6.7 mg.
-
pH: 6.0.
Prefilled Syringe:
-
1 mL, single-dose.
-
Gauge: 27 gauge.
-
Needle: Half-inch.
-
Components per mL:
-
Belimumab 200 mg.
-
L-arginine hydrochloride 5.3 mg.
-
L-histidine 0.65 mg.
-
L-histidine monohydrochloride 1.2 mg.
-
Polysorbate 80 (0.1 mg).
-
Sodium chloride 6.7 mg.
-
pH: 6.0.
Mechanism of Action:
Belimumab is an inhibitor of BLyS - it blocks the binding of soluble BLyS to its receptors present on B-cells. This results in:
-
Inhibition of B-cell survival, including autoreactive B-cells.
-
Limited differentiation of B-cells into immunoglobulin-producing plasma cells.
Pharmacodynamics:
Treatment with Belimumab in adult patients causes the following changes:
-
Reduction in circulating CD19+, CD20+.
-
Reduction in naive and activated B-cells.
-
Reduction in SLE B-cell subset.
-
An initial increase in memory cells is followed by a baseline-level decline towards Week 52.
-
Reduction in IgG and anti-double-stranded DNA antibodies (anti-dsDNA).
-
Increase in complement C3 and C4.
In Lupus Nephritis Patients:
-
An initial decrease in serum IgG is followed by an increase in IgG and decreased proteinuria.
-
Reduction in circulating B-cells and B-cell subsets.
-
Reduction in autoantibodies.
-
Increase in complements.
Pharmacokinetics:
Intravenous Infusion:
Pharmacokinetic Parameters in Adult SLE Patients After IV Infusion of Belimumab 10 mg/kg:

Lupus Nephritis:
-
Belimumab exposure was lower than seen in SLE studies - seen in patients with higher proteinuria.
-
When proteinuria was decreased to ≤1 g/g - Belimumab exposure and clearance were similar to SLE patients.
Pharmacokinetic Parameters in Adult SLE Patients After Subcutaneous Injection of Belimumab 200 mg Once Weekly:
-
Time to reach maximum serum concentration (Cmax): 2.6 days (Tmax) after administration at a steady state.
-
Bioavailability of Belimumab: 74 %.

Lupus Nephritis:
Steady-state average concentrations of Belimumab 200 mg (SC) once weekly in adults with lupus nephritis are predicted to be similar to concentrations in adults receiving IV Belimumab 10 mg/kg every four weeks for lupus nephritis.
Special Considerations:
- Age: No significant change was observed in the pharmacokinetics of Belimumab (both IV and SC administration) in relation to patients aged 18 to 45 years.
- Geriatric Patients: Data on elderly patients (over 65 years) is limited and pharmacokinetic details are not well-cataloged.
- Pediatric Patients: Belimumab exposures (Belimumab dosage: 10 mg/kg IV) in pediatric patients with SLE are similar to that of adult SLE patients.
- Gender: There were no significant differences in Belimumab pharmacokinetics between males and females. However, the trial populations in both IV and SC administration were predominantly female, which may indicate a skewed perspective as the data on the effects of Belimumab in men appears insufficient.
- Race: Race does not significantly influence Belimumab pharmacokinetics. However, studies have shown that the efficacy and safety of Belimumab in black patients is less well-established, and may require caution.
- Renal Impairment: Belimumab administered in patients with mild, moderate, and severe renal impairment has not caused adverse effects and has been reported safe.
- Hepatic Impairment: No formal trials have been conducted on patients with hepatic impairment, but the baseline ALT and AST levels have not been known to influence the pharmacokinetics of Belimumab.
- Weight: Weight does not appear to have relevance to the pharmacokinetic effects of Belimumab. Dose adjustments are not required.
Drug Interaction Studies:
Formal studies on drug interactions have not been conducted for Belimumab. However, the concomitant use of certain drugs has not given rise to adverse reactions. These drugs include:
-
Cyclophosphamide.
-
Mycophenolate.
-
Antimalarials.
-
Aspirin.
-
Azathioprine.
-
Methotrexate.
-
NSAIDs.
-
HMG-CoA reductase inhibitors.
Coadministration of steroids and angiotensin-converting enzyme (ACE) inhibitors caused a clinically insignificant increase in systemic clearance of Belimumab.
Clinical Trials:
- IV Infusion:
- Systemic Lupus Erythematosus (SLE):
The Belimumab in Subjects With Systemic Lupus Erythematosus -76 (BLISS-76) Trial (Phase III) evaluated the safety and efficacy of Belimumab in patients with SLE when added to standard therapy.
-
Study Design:
-
Type of Study: Multicenter, randomized, double-blinded, placebo-controlled trial (Phase III).
-
Study Duration: 76 weeks.
-
Methods: Seropositive patients (n= 819) were randomized to one of three groups:
-
Belimumab 10 mg/kg.
-
Belimumab 1 mg/kg.
-
Placebo.
All patients were also given standard therapy. IV infusion of Belimumab was given on Days 0, 14, 28 -followed by every 28 days during the entire study period (76 weeks).
-
Primary Outcome Measure: Improvement in patient response rates [measured by SLE Responder Index (SRI)] at Week 52.
-
Secondary Outcome Measure: Improvement in patient response rates [measured by SLE Responder Index (SRI)] at Week 76.
-
Results:
-
Belimumab 1 mg/kg V/S Placebo: No significant difference in SRI (40.6 % v/s 33.5 %, p=0.089).
-
Belimumab 10 mg/kg V/S Placebo:
-
Week 52: Significant difference noted in SRI (43.2 % v/s 33.5 %, p=0.017).
-
Week 76: No significant difference - change in SRI not sustained (32.4 % with placebo, 39.1 % with Belimumab 1 mg/kg, and 38.5 % with Belimumab 10 mg/kg).
-
Adverse Events Profile:
Rates of adverse events were similar to that of the placebo group. These included:
-
Infections.
-
Malignancies.
-
Death.
Lupus Nephritis: The Belimumab International Study in Lupus Nephritis (BLISS-LN Trial, Phase III) evaluated the safety and efficacy of Belimumab in patients with lupus nephritis when added to standard therapy.
-
Study Design:
-
Type of Study: Multinational, multicenter, randomized, double-blinded, placebo-controlled trial (Phase III) conducted across 107 sites in 21 countries.
-
Study Duration: 104 weeks.
-
Methods: Adults with biopsy-proven, active lupus nephritis were randomized in a 1:1 ratio (n=448) into the following groups:
-
Belimumab 10 mg/kg.
-
Placebo.
All patients were also given standard therapy. IV infusion of Belimumab or placebo was given on Days 1, 15, 29 -followed by every 28 days during the entire study period (104 weeks).
-
Primary Outcome Measure:
Primary Efficacy Renal Response: This was measured as:
-
Urinary protein: creatinine ratio = ≤0.7.
-
The estimated glomerular filtration rate [eGFR] is no worse than 20 % below the value before the renal flare (pre-flare value) or ≥60 ml per minute per 1.73 m2 of body-surface area.
-
No point in rescue therapy.
-
Secondary Outcome Measure:
Complete Renal Response: This was measured as:
-
Urinary protein: creatinine ratio = <0.5.
-
eGFR is no worse than 10 % below the pre-flare value or ≥90 ml per minute per 1.73 m2.
-
No point in rescue therapy.
-
Results:
Week 104: Significant differences in primary efficacy renal response and complete renal response in the Belimumab group compared to the placebo group.
-
Primary Efficacy Renal Response: 43 % vs. 32 %; odds ratio, 1.6; 95 % confidence interval [CI], 1.0 to 2.3; P=0.03.
-
Complete Renal Response: 30 % vs. 20 %; odds ratio, 1.7; 95 % CI, 1.1 to 2.7; P=0.02.
Renal-Related Event or Death Risk: Lower in Belimumab patients compared to the placebo group (hazard ratio, 0.51; 95 % CI, 0.34 to 0.77; P=0.001).
-
Safety Profile of Belimumab: Consistent with previous trials.
-
Subcutaneous Injection (SC Injection):
SLE: The BLISS-SC Trial (Phase III) evaluated the efficacy and safety of SC Belimumab in SLE patients receiving standard therapy over 52 weeks.
-
Study Design:
-
Type of Study: Multinational, multicenter, randomized, double-blinded, placebo-controlled study (Phase III) conducted across 177 sites in 30 countries.
-
Study Duration: 52 weeks.
-
Methods: Moderate‐to‐severe SLE patients [≥8 scores on the Safety of Estrogens in Lupus Erythematosus National Assessment (SELENA) version of the SLE Disease Activity Index (SLEDAI), n=836] were randomized 2:1 to the following groups:
-
Belimumab 200 mg once weekly.
-
Placebo group.
Prefilled syringes were used for drug administration. All patients were also given standard SLE therapy.
-
Primary Outcome Measure:
-
SLE responder index (SRI4) at week 52.
-
Secondary Outcome Measure:
-
Reduction in corticosteroid dosage.
-
Reduction in time to severe flare.
-
Safety Assessment: In accordance with reported adverse events and laboratory test results.
-
Results:
-
SELENA-SLEDAI Scores at Entry:
-
Belimumab Group: 10.5.
-
Placebo Group: 10.3.
-
Primary Endpoint Result: The number of Belimumab patients who were SRI4 responders exceeded the placebo group patients (61.4 % versus 48.4 %; odds ratio [OR] 1.68 [95 % confidence interval (95 % CI) 1.25–2.25]; P = 0.0006).
-
Secondary Endpoint Results:
-
Belimumab group patients showed improved time to and risk of severe flare (median 171.0 days versus 118.0 days; hazard ratio 0.51 [95 % CI 0.35–0.74]; P = 0.0004).
-
The number of Belimumab group patients who could reduce corticosteroid dosage exceeded the placebo group patients - reduction in corticosteroid dosage by ≥25 % (to ≤7.5 mg/day) during weeks 40–52 (18.2 % versus 11.9 %; OR 1.65 [95 % CI 0.95–2.84]; P = 0.0732).
-
Adverse Events Profile:
Comparable between both groups.
-
Serious Adverse Events Reported:
-
Belimumab Patients: 10.8 %.
-
Placebo Patients: 15.7 %.
-
Worsening of IgG Hypoglobulinemia by ≥2 Grades:
-
Belimumab Patients: 0.9 %.
-
Placebo Patients: 1.4 %.
Clinical Trials for Other Therapeutic Uses of Belimumab:
Ongoing clinical trials on Belimumab have considered an array of possible uses of Belimumab in other autoimmune disorders, including:
-
Rheumatoid arthritis.
-
Idiopathic thrombocytopenic purpura.
-
Myasthenia gravis.
-
Systemic sclerosis.
-
Sjogren's syndrome.
Belimumab as Prophylaxis:
A Phase 1 study has also demonstrated the possibility of using Belimumab as a prophylactic therapy to prevent chronic graft-versus-host disease in adult patients undergoing allogeneic transplantation.
-
Study Design:
-
Type of Study: Single-center, investigator-initiated phase 1 trial.
-
Study Population: 9 adult patients with hematologic malignancies (who were in remission). The malignancies included:
-
Acute myeloid leukemia (AML).
-
Acute lymphocytic leukemia (ALL).
-
Myelodysplastic syndromes (MDS).
-
Lymphoma.
-
Methods:
Patients received Belimumab (10 mg/kg) every two weeks for three doses. This was followed by four doses (one per month).
Initiation of Treatment: 50 days to 80 days after allogeneic hematopoietic cell transplantation.
-
Results:
Belimumab appeared to be well-tolerated and was not linked to severe infections or myelosuppression (suppressed bone marrow activity, leading to fewer red blood cells, white blood cells, and platelets). Eight out of nine patients received all seven doses of Belimumab in accordance with pre-planned parameters. Five patients are alive after over 20 months of follow-up with no signs of chronic graft-versus-host disease (cGVHD).
Patient Counseling Information:
- Advise patients to acquaint themselves with the patient labeling information provided by the FDA (Medication Guide and Instructions for Use).
- Advise patients of the effects they may notice with Belimumab, including:
-
Reduced ability to fight infections.
-
Rare possibility of neurological symptoms such as:
-
Confusion.
-
Vision problems.
-
Memory loss.
-
Loss of balance.
-
Dizziness.
-
Hypersensitivity reactions include:
-
Wheezing.
-
Breathing difficulty.
-
Rashes.
-
Muscle ache.
-
Hypotension.
-
Bradycardia.
-
Facial swelling.
-
Nausea.
-
Fatigue.
-
Increase in symptoms of depression and suicidal ideation.
-
Impaired response to living vaccines - advise against taking a live vaccine for 30 days before and during the course of treatment with Belimumab.
-
Impaired immune system possibility in infants of treated mothers - advise them to inform the doctor of pregnancy (whether suspected or confirmed).
Additional Information:
- Inform patients of pregnancy registries to monitor the fetal outcomes of pregnant women (if available in your country).
- Inform patients of what to do in case of overdose, missed dose, or adverse conditions like suicidal thoughts and other physical illnesses resulting from Belimumab.
- Provide patients with information on local poison control services, suicide hotlines, and other essential information.












