Overview:
Entrectinib is an oral selective inhibitor of neurotrophic T receptor kinase (NTRK) and ROS1 of the c-ros oncogene 1 (located on the long arm of chromosome 6). It treats solid tumors with NTRK gene fusion and non-small cell lung cancer with ROS1 mutations. The Food and Drug Administration (FDA) granted accelerated approval to use Entrectinib for adults and pediatric patients in August 2019. The medication can be given to adults 12 years and older with solid tumors with neurotrophic tyrosine receptor kinase (NTRK) gene fusion without a known acquired resistance mutation and metastatic non-small cell lung cancer therapy.
How Does Entrectinib Work?
Cancers with NTRK fusion or ROS1 gene fusion produce abnormal proteins that cause an uncontrolled increase of cancer cells. Entrectinib blocks the action of these proteins and so prevents the increase in cancer cells, thereby slowing cancer growth. It may also affect normal cells, which can cause side effects.
Uses of Entrectinib:
-
Entrectinib is given in treating metastatic ROS1-positive, non-small cell lung cancer (NSCLC) in adults and children above 12 years of age where it has spread to other body parts.
-
The drug is used to treat NTRK gene fusion-positive solid tumors, which have spread or may require surgical resection, which can result in severe morbidity. It may be used to treat solid tumors for which no comparative alternative treatment is available.
-
Entrectinib can treat certain types of solid tumors that have worsened with other chemotherapy medications.
-
The drug offers more benefits over similar anaplastic lymphoma kinase (ALK) inhibitors, such as Alectinib, Ceritinib, and Lorlatinib. It is because of its wide range of targets.
Limitations:
-
Entrectinib targets TRK fusion proteins inside cancer cells but may also affect the normal cells, causing side effects.
-
Liver test abnormalities are frequent with Entrectinib. The drug is reported to cause an alanine transaminase (ALT) elevation of up to 38 % in trials.
Dosage:
1. Route of Administration - Oral
2. Dosage Forms -
-
100 mg - Hard capsules size 2, yellow, opaque with 100 imprinted on the body.
-
200 mg - Hard capsule, size 0, orange, opaque with 200 imprinted on the body.
3. Dosage Strengths -
-
100 milligrams.
-
200 milligrams.
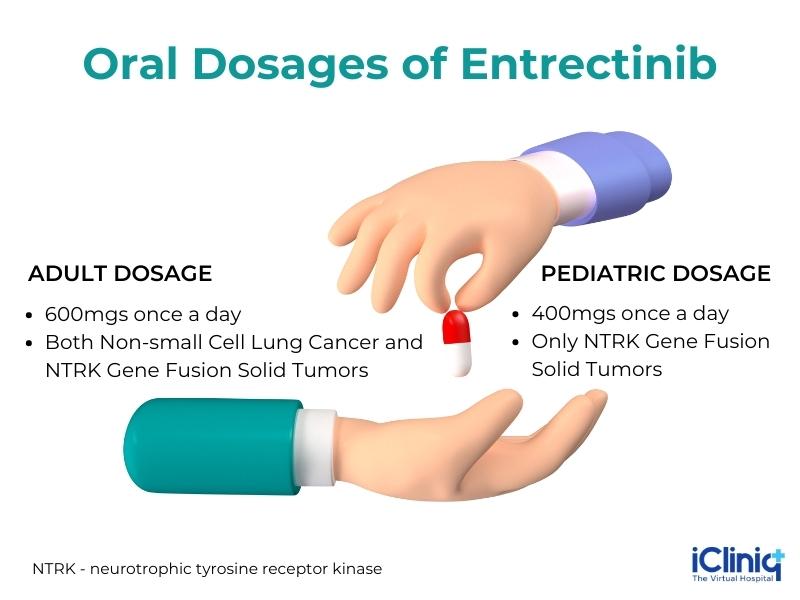
3. Typical Dosage -
4. Dosage Modification -
For Adverse Reactions -
-
First Dose Reduction - 400 milligrams per day.
-
Second Dose Reduction - 200 milligrams per day.
For Heart Failure -
-
Grade 2 or 3 - Withhold until recovers to grade 1. Resume at a reduced dose.
-
Grade 4 - Discontinue permanently.
Central Nervous System Effects -
-
Intolerable grade 2 - Withhold until recovers to grade 1.
-
Grade 3 - Withhold until recovers to grade 1. Resume at a reduced dose.
-
Grade 4 - Discontinue permanently.
Hepatotoxicity -
-
Grade 3 - Withhold until recovers to grade 1.
-
Elevated ALT / AST (alanine transaminase and aspartate transaminase) - Discontinue permanently.
Special Considerations
-
Pregnancy- Using Entrectinib during pregnancy can harm the unborn baby. Female patients of reproductive potential should use contraceptives during the treatment with Entrectinib for five weeks following the final dose.
-
Lactation - There are inadequate studies on women lactating infants using Entrectinib.
-
Pediatric Population - Therapy with Entrectinib should be given with caution to small children, as the drug increases the risk of fractures.
-
Geriatric Patients - Entrectinib should be administered cautiously in older adults, as they have age-related problems. Also, the medication increases the risk of fractures and should be given cautiously.
-
Renal Impairment - Entrectinib is not studied in patients with a history or suffering from renal impairment.
-
Hepatic Impairment - No studies are available on the effect of Entrectinib in patients with severe hepatic impairment.
-
Alcohol - Drinking alcohol should be avoided while taking Entrectinib therapy. Avoid driving and operating heavy machinery while taking Entrectinib.
Warnings and Contraindications:
Contraindications:
-
Hypersensitivity - Avoid treatment with Entrectinib if the person develops allergic reactions, such as hives, difficulty in swallowing or breathing, or swelling in the neck, throat, or tongue.
-
Serious Eye Problems - Therapy with Entrectinib causes sudden vision loss, blurred vision, tunnel vision, eye pain, or swelling. Therapy with Entrectinib should be avoided if possible.
-
Pregnancy and Breastfeeding - Treatment with Entrectinib is not recommended during pregnancy, as it may harm the fetus. Also, because of its potential adverse reactions in breastfed children, breastfeeding is not recommended during treatment with Entrectinib and for seven days after the final dose.
Warnings and Precautions:
-
Congestive Heart Failure - Monitor the patients for signs and symptoms that have a known risk for congestive heart failure before initiation of Entrectinib. Assess the left ventricular ejection fraction in patients with the help of cardiac biopsy or magnetic resonance imaging (MRI).
-
Central Nervous System Effects - Therapy with Entrectinib causes a broad spectrum of central nervous system adverse reactions, such as cognitive impairment (trouble in learning or concentrating), mood disorders (depression or bipolar disorders), dizziness, and disturbances in sleep.
-
Skeletal Fractures - Treatment with Entrectinib increases the risk of fractures. Patients must be promptly evaluated with signs or symptoms, such as pain, change in mobility, or deformity.
-
Hepatotoxicity - There is an increased risk of liver damage, infection, and toxicity in patients receiving Entrectinib. Monitor liver tests every two weeks, including ALT and AST, during the first month of treatment with Entrectinib.
-
QT Interval Prolongation - Patients who have or are at significant risk of developing QTc interval prolongation, including those with known long QT syndrome, should be assessed and monitored periodically. These patients may require reduced doses or permanent discontinuation based on their QTc interval prolongation severity.
-
Vision Disorders - Vision disorders, such as blurred vision, photophobia, diplopia, or a visual impairment, have been seen across clinical trials on patients receiving Entrectinib. Dose adjustment may be required until improvement or stabilization is achieved.
For Patients
What Is Metastatic Non-Small Cell Lung Cancer (NSCLC)?
Metastatic non-small cell lung cancer is an advanced stage that has spread to distant body parts. Cancer spreads beyond the lungs through the lymphatic system (that balances the body fluids and defends the body against infections) and blood. It can spread to any organ or tissue in the body, such as bone, brain, liver, or adrenal glands. Smoking is one of the major risk factors for non-small cell lung cancer. Other factors may include old age, exposure to secondhand smoke, asbestos, arsenic, chromium, and beryllium, radiation exposure, air pollution, those with a history of lung cancer in their family, or being infected with human immunodeficiency virus (HIV).
NSCLC spreads in four stages, with stage four being the most critical. It occurs when the cancer cells spread to areas in the chest and other body parts.
Symptoms of Metastatic Non-Small Cell Lung Cancer:
The signs and symptoms of non-small cell cancer are usually found during a chest X-ray for another condition. The symptoms may include
-
Bone pain.
-
Dizziness.
-
Balancing issues.
-
Yellowing of the skin and eyes.
-
Swelling of lymph nodes in the neck or near the collarbone.
Other common symptoms of NSCLC include
-
Trouble breathing.
-
Persistent cough.
-
Unexplained weight loss.
-
Hoarseness.
-
Chest discomfort.
-
Difficult swallowing.
-
Swelling in the face or neck.
Some lung cancers can cause groups of symptoms, such as
-
Horner Syndrome - The condition is characterized by drooping or weakness of one upper eyelid, pupils, and no sweat on one side of the body.
-
Superior Vena Cava Syndrome - It causes swelling in the face, neck, arms, and upper chest, leading to headaches and unconsciousness.
-
Paraneoplastic Syndrome - A condition when the hormones travel in distant sites through blood.
Why Is Entrectinib Used for Metastatic Non-small Cell Lung Cancer?
Treatment with Entrectinib gives a continued clinical benefit to patients with ROS1-positive non-small cell lung cancer, including those with central nervous system metastases. The drug is active with disease control for a long duration in patients with ROS1 fusion-positive NSCLC and with a well-tolerated and managed safety profile.
Facts One Should Know About Entrectinib:
-
Entrectinib causes drowsiness and dizziness. Changes in vision, confusion, tiredness, and memory loss. The drug should be avoided while driving or operating heavy machinery until the symptoms resolve.
-
Entrectinib is used only if cancer has a specific abnormal gene. The doctor will test for this gene before prescribing the drug.
-
Entrectinib can cause high uric acid levels. In case of joint pain or swelling, inform the doctor.
-
Men should take contraception for at least three months after the final dose and women for at least five weeks following the final dose of Entrectinib.
How Should One Take Entrectinib?
-
Entrectinib may be taken with food or without food.
-
Entrectinib capsules should not be chewed or crushed.
-
In case a person vomits soon after taking Entrectinib, take another dose as soon as possible on the same day.
-
Do not double the dose of Entrectinib, as it can cause serious adverse effects.
-
Do not share the drugs with others.
Information To Be Given to the Doctor Before Taking Entrectinib:
-
Allergies- Inform the doctor if one is allergic to Entrectinib or its ingredients. The doctor must be briefed on other allergies to food, dyes, or other substances.
-
Medical History- Inform the doctor of any concurrent illnesses one may be suffering from, especially brain-related damages such as head injury, cancer, seizures, prolonged breathing problems such as asthma, sleep apnea, obstructive pulmonary disease, kidney disease, liver disease, mental disorders such as confusion, depression, suicidal thoughts, or history of substance abuse.
-
Drug History- Inform the doctor about any other drugs or over-the-counter drugs one may be taking. These drugs may interact with Entrectinib and cause adverse effects.
-
Surgery - Before starting Entrectinib, inform the doctor if one has recently had any surgery or dental appointment.
-
Pregnancy- Entrectinib can cause harm to the fetus. A pregnancy test must be done before starting the drug.
-
Lactation - The doctor may advise stopping breastfeeding till the treatment with Entrectinib is completed.
-
Geriatrics - Treatment with Entrectinib should be given with caution in older adults, as it causes various nervous system problems, such as confusion, dizziness, memory loss, hallucinations, or suicidal thoughts.
-
Children - Therapy with Entrectinib should be administered cautiously in children, as it can cause a high risk of side effects.
Safety of Entrectinib:
In multiple studies, Entrectinib is safe for treating patients with locally advanced or metastatic ROS1 fusion-positive NSCLC. This led to FDA approval of Entrectinib in these patients in August 2019. The drug is well tolerated and induces clinically meaningful, durable systemic and intracranial responses in patients with NTRK-fusion-positive solid tumors, including those with NSCLC.
Effectiveness of Entrectinib:
Entrectinib is a central nervous system-active, potent, and selective inhibitor of ROS1 and TRKA (tyrosine kinase inhibitors). The drug has a good blood-brain-barrier penetration in contrast to other anti-cancer drugs. Entrectinib is the first line of treatment for ROS1 mutations, especially in patients without brain metastases.
Side Effects Expected With Entrectinib:
-
Tiredness.
-
Constipation.
-
Dizziness.
-
Nausea.
-
Diarrhea.
-
Change in taste.
-
Abnormal touch sensation.
-
Muscle pain.
-
Vomiting.
-
Cough.
-
Joint pain.
-
Vision changes.
-
Weight gain.
-
Difficulty with learning, memory, or problem-solving.
-
Confusion.
-
Fever.
Can One Stop Taking Entrectinib Suddenly Without the Doctor's Approval?
-
Never stop taking medicines without talking to the doctor. The doctor will probably decrease the dose gradually.
-
Stopping therapy with Entrectinib suddenly can cause undesired adverse effects and recurrence of the tumor.
-
Do not take the drug more than advised by the doctor.
Dietary Restrictions to Consider When Taking Entrectinib:
-
Grapefruit - When taking Entrectinib, one should avoid consuming grapefruit juice or eating grapefruit. It can increase the amount of Entrectinib in the blood to a harmful level.
-
Diet - Eat a healthy balanced diet. Adding fibers, such as green vegetables, can help to manage side effects like constipation.
-
Alcohol and Smoking - Avoid drinking alcohol, as it can exacerbate the central nervous system side effects, such as drowsiness, confusion, or lightheadedness. Smoking should be avoided while taking Entrectinib.
Storage of Entrectinib:
-
Keep Entrectinib in its original packing at room temperature.
-
Direct contact with heat, air, and light may damage the medicines. Therefore, keep the medicines away from direct light and heat.
-
Keep the Entrectinib capsules safe and out of reach of children and pets. Always lock the safety caps of the medication to protect small children from poisoning themselves.
Disposal of Entrectinib:
-
Do not keep outdated medicines or medicines that are no longer needed.
-
Likewise, unneeded medicine solutions should not be disposed of by flushing or throwing them out with regular garbage.
-
Dispose of the medicine through the local medicine take-back program, which can be accessed or learned more about through the local pharmacist.
-
Some people may not have access to a drug take-back program. Ask the local pharmacist about any other drug disposal options available.
Overdose:
If a person has taken an overdose of Entrectinib, seek emergency medical attention or call the helpline number. If a person collapses, has had a seizure, or has trouble breathing, immediately call the doctor.
For Doctors
Indications
-
Entrectinib is indicated in the treatment of adult patients having ROS1-positive metastatic non-small cell lung cancer.
-
It can treat non-small cell lung cancer regardless of its location.
-
Entrectinib can be used if the patient has not previously received treatment with NTRK inhibitors and cancer has metastasized.
What Is the Pharmacology of Entrectinib Injection?
Description
Entrectinib is a kinase inhibitor that blocks the action of the abnormal proteins that signal cancer. The drug is an orally bioavailable inhibitor of tyrosine kinase tropomyosin receptor kinases.
Active Ingredients
Entrectinib.
Inactive Ingredients
-
Tartaric acid.
-
Lactose anhydrous.
-
Hypromellose.
-
Crospovidone.
-
Microcrystalline cellulose.
-
Colloidal Silicon dioxide.
-
Magnesium stearate.
Clinical Pharmacology:
Mechanism of Action
Entrectinib is a tyrosine kinase inhibitor that acts on several receptors. The drug acts as an ATP competitor to inhibit tropomyosin receptor tyrosine kinases (TRK) and proto-oncogene tyrosine-protein kinase ROS1 and anaplastic lymphoma kinase (ALK). TRK receptors produce cell proliferation through downstream signaling of the mitogen-activated protein kinase, phosphoinositide 3-kinase, and phospholipase C. ALK produces similar signaling with downstream JAK activation. The inhibition of the pathways suppresses the proliferation of cancer cells and shifts the balance in favor of apoptosis, resulting in the shrinking of tumor volume.
Pharmacodynamics
Entrectinib and its active metabolite suppress pathways contribute to cell survival and proliferation. The suppression shifts the balance in favor of apoptosis (a series of molecular steps leading to cell death), thereby preventing cancer cell growth and shrinking tumors.
Pharmacokinetics
-
The Mean Cmax - Four to six hours.
-
Median Tmax- Four to Five hours after administration of a single 600-milligram dose. The presence of food has no significant effect on the extent of absorption.
[Cmax- Maximum concentration achieved by a drug in the blood, cerebrospinal fluid, or target organ after administration of a dose].
[Tmax- Time taken for a drug to reach maximum concentration after administration of a dose].
Pharmacokinetic Changes-
A. Distribution
-
Mean Half-life - 20 hours (half-life of M5 - 40 hours).
-
Terminal Half-Life in Plasma - Ratio between Entrectinib and M5 were 1.89 and 2.01, respectively.
-
Time to Approach Steady-state Levels - Within two weeks.
-
Mean Volume of Distribution - 551 liters. The active metabolite of Entrectinib, M5, has an apparent volume of distribution of 81.1 liters.
-
Plasma Binding - 99 % bound to plasma protein.
B. Metabolism
-
Metabolic Processes- CyP3A4 is responsible for 76 % of Entrectinib metabolism. This includes metabolism to the active metabolite M5, which has similar pharmacological activity to the parent drug and exists at approximately 40 % of the steady-state concentration of Entrectinib.
C. Elimination:
-
Feces - 83 % (36 % was Entrectinib and 22 % M5).
-
Urine - 3 %.
Apparent clearance of Entrectinib is 19.6 liters per hour, while the apparent clearance of active metabolite is 52.4 liters per hour.
Special Considerations
-
Renal Impairment - The effect of renal impairment of Entrectinib after administration has not been studied. However, the drug must be given with caution in patients with preexisting renal impairment.
-
Hepatic Impairment - Mild and transient elevations in liver enzymes may occur in patients receiving Entrectinib. However, clinically apparent hepatotoxicity is rare.
-
Alcohol - Do not drink alcohol while on Entrectinib. The combination can raise the risk of liver damage.
Drug Interactions
-
Adenosine - Co-administration of Entrectinib and Adenosine increases irregular heart rhythm. It can cause severe and life-threatening consequences.
-
Proton Pump Inhibitors - Combining Entrectinib with proton pump inhibitors (PPI), such as Lansoprazole, can reduce the drug's effectiveness.
-
Digoxin - Coadministration of a single dose of Entrectinib with Digoxin increased the absorption of the drug by 28 %, causing an increased risk of side effects.
-
CYP3A Inducers - Combining Rifampicin with a single dose of 600 milligrams can affect the absorption and effectiveness of the drug.
-
Amiodarone - Using Amiodarone with Entrectinib can increase the risk of an irregular heart rhythm that may be critical and potentially life-threatening.
-
Aprepitant - Using an Aprepitant may significantly increase the blood levels of Entrectinib and cause an increased risk of side effects, such as dizziness, confusion, and mood changes.
What Have Clinical Trials Shown About Entrectinib?
Trial 1:
Objective - To explore the use of Entrectinib capsules in patients with locally advanced or metastatic ROS1 fusion-positive NSCLC.
-
An integrated analysis of three phases of one or two trials of Entrectinib.
-
The efficacy-evaluable population included adult patients above 18 years with local advanced ROS1 fusion-positive NSCLC who received Entrectinib at a dose of 600 milligrams orally per day for at least 12 months.
-
All patients with an Eastern Cooperative Oncology Group status of o-2 and previous cancer treatment.
Primary End Point - The number of patients with an objective response (complete or partial response according to Response Evaluation Criteria in Solid Tumors version 1.1) and duration of response were evaluated by blinded independent central review.
Findings - 59 % of patients exhibit grade 1 or 2 treatment-related side effects, and 34 % had grade 3 or 4 treatment-related adverse effects, the most common being weight increase and neutropenia. No treatment-related deaths occurred.
Results - Entrectinib is an active component with effective disease control in patients with ROS1 Fusion-positive NSCLC is well tolerated with a manageable safety profile, making it amenable to long-term dosing in these patients. The data stresses the need to test for ROS routinely. Fusions to broaden therapeutic options for patients with ROS1 fusion-positive NSCLC.
Trial 2:
Objective - A study for comparing the efficacy and safety of Entrectinib and Crizotinib in participants with advanced metastatic ROS1 non-small cell lung cancer (NSCLC), with or without central nervous system metastases.
-
The participants self-administered oral Entrectinib or Crizotinib, or as described in accordance with the protocol and local prescribing information.
-
Treatment will continue until progressive disease, unacceptable toxicity, or withdrawal from the study.
-
A interventional clinical trial with 220 participants was randomized by giving Entrectinib 600 mg once a day and Crizotinib (250 mg) twice a day.
Primary Outcome - Progression-free survival in participants with central nervous system metastases at baseline for up to seven years.
Secondary Outcome - Stipulated treatment comparison versus real-world evidence. No significant trends were recorded between the endpoint values.
Result - Crizotinib and Entrectinib have comparable efficacy in ROS1-positive non-small cell lung cancer.
Patient Counseling Information
Administration Instructions -
-
Entrectinib is usually taken with or without food once a day.
-
Take the medication at the same time each day.
-
Do not switch doses or stop taking Entrectinib without the doctor's consent.
-
If one vomits shortly after taking Entrectinib, take another dose.
-
Entrectinib can affect healthy cells as well as cancer cells. Report any side effects.
-
Do not receive any kind of immunization or vaccination without talking to the doctor
-
Periodic blood work must be done to monitor the complete blood count and liver function tests.












