Introduction:
Vilanterol is used in combination with several other medications. For example, a combination of Vilanterol and Fluticasone furoate is used to manage asthma and COPD. It is an ultra-long-acting beta 2 adrenoreceptor agonist to improve asthma symptoms and prevent bronchospasm and asthma attacks. Vilanterol is a novel, long-acting and revolutionary drug because it is chemically distinct from the other drugs used to treat asthma.
The biggest advantage of Vilanterol over other antiasthmatic drugs is that they require twice-daily dosing, whereas Vilanterol needs to be taken once daily. In addition, the drug has shown to exhibit faster onset and longer duration of action in the lung tissues of the human body. Clinical trials have demonstrated the 24-hour efficacy of Vilanterol in patients with long-standing asthma and are administered concurrently with inhaled corticosteroids daily.
Developmental History and Drug Approval Timeline:
Vilanterol was originally developed and manufactured by GlaxoSmithKline and Theravance, Inc. The safety and efficacy of the drug were evaluated in a clinical trial involving 12,000 patients 12 years of age and above. Also, a series of double-blind placebo-controlled trials were done on patients to measure the safety and efficacy of this drug against asthma and COPD. Based on these trial results, the US Food and drug administration (FDA) approved Vilanterol for the treatment of COPD on 10th May 2013. Next, the FDA updated another indication of the drug on 30th April 2015 which stated that Vilanterol could also be used for asthma in individuals 18 years of age or above.
How Does Vilanterol Work to Treat Asthma and COPD?
Vilanterol and Fluticasone furoate is used as a dry powder combination for the long-term maintenance treatment of airflow obstruction and for reducing flare-ups or exacerbations in patients suffering from COPD. Vilanterol belongs to the class of medications called long-acting beta-antagonists that work by relaxing and opening the air passages in the lungs. When a patient has COPD and asthma symptoms flare up, their airways swell. As a result, these patients find it difficult to breathe.
In addition, the patient might have the following problems due to the narrowed airways:
-
Muscle twitches.
-
The buildup of the fluid or sputum in the lungs.
-
Tightening of the blood vessels present in the lungs.
When Vilanteol is administered with Fluticasone furoate, it activates the enzyme that increases the concentration of cyclic-3',5'-adenosine monophosphate (cAMP). This enzyme relaxes the bronchial muscles, reduces the releases of inflammatory cell mediators from the mast cells, and relieves bronchospasms. As a result, the patient can breathe easily.
What Is Vilanterol Used For?
The FDA has approved Vilanterol for use in combination with Fluticasone furoate to treat airflow obstruction in patients with asthma and COPD. It is also indicated for once-daily maintenance of asthma and COPD in patients 18 years of age or above and having reversible obstructive airway disease.
The uses have been described below:
-
Vilanterol in Asthma - Vilanterol has been approved to prevent asthma in adults who:
-
Have uncontrolled asthma even after taking long-term asthma medications.
-
Require treatment with long-acting beta 2 adrenergic agonists and inhaled corticosteroids.
-
-
Vilanterol in COPD - Vilanterol helps the lungs function properly in COPD patients. This is because COPD is a group of conditions that affect the lungs, including emphysema and chronic bronchitis. Vilanterol helps the patient breathe by relaxing the airway muscles so they remain open. As per the study reports, this drug has also been approved to reduce the number of COPD flare-ups.
What Makes Vilanterol Unique?
Vilanterol is an inhalation drug that is used in combination with Fluticasone furoate to improve the airflow in patients with asthma and COPD. During the in vitro and in vivo studies, it was noted that the drug blocked pro-inflammatory factors and the exaggerated antigen-induced immune response in the lungs. In addition, it has a unique capacity of binding to the human glucocorticoid receptors, which is 30 times more than other drugs like Dexamethasone. Also, this drug directly increases the concentration of the enzyme responsible for relaxing the bronchial smooth muscles.
Dosage and Administration:
Vilanterol must be administered as a single dose daily by the orally inhaled route. Therefore, it must be used at the same time daily and should not be used more than once every 24 hours. After inhaling the drug, the patient must rinse his mouth with water without swallowing to overcome the risk of oropharyngeal candidiasis. The patient must keep in mind that the drug must not be inhaled repeatedly during the day. This is because some patients might experience side effects with higher doses. In addition, patients taking Vilanterol must not use other long-acting beta-agonists for asthma and COPD.
Dosage for Chronic Obstructive Pulmonary Disease:
Vilanterol 100/25 should be administered as an inhalation dose once daily. The maximum recommended dosage is one inhalation of Vilanterol 100/25, and it is the only strength indicated for the management of COPD. If the patient experiences shortness of breath between the doses, an inhaled short-acting beta 2-agonist or a rescue medication must be taken for immediate relief.
Dosage for Asthma:
The recommended starting dosage for asthma is Vilanterol 100/25 or 200/25, but it must be administered as one inhalation once daily. The doctor must consider the severity of the patient’s condition depending on the previous medical and asthma therapy history. They must also monitor the dosage of inhaled corticosteroids, the control of asthma symptoms and the risk of future exacerbations. The median time of onset of the drug was noted to be 15 minutes after starting the treatment.
However, the median time of onset and the degree of symptom relief will vary from one patient to another. People who do not respond to Vilanterol 100/25 can be administered a higher dose, Vilanterol 200/25, for additional improvements in the asthma symptoms. If asthma symptoms exacerbate between the doses, a rescue drug like Albuterol can be taken for immediate relief. If the patient is already taking Vilaterol but has not achieved the desired results, the doctor must reevaluate the therapeutic regimen, and additional therapeutic options must be considered.
The following alternative therapeutic options can be considered:
-
Increasing the dosage strength of Vilanterol.
-
Use of additional inhaled corticosteroids.
-
Initiating oral corticosteroid therapy.
Information About Asthma and COPD for Patients:
What Are Asthma and COPD?
Asthma is a non-communicable respiratory disease in which the airways swell and narrow due to the deposition of extra mucus. As a result, the patient experiences difficulty breathing, which triggers coughing, whistling, or wheezing while a person breathes and shortness of breath. COPD is a long-standing inflammatory disease that obstructs the airflow from the lungs. COPD is not a single condition, rather; it is a group of conditions that affects the respiratory system. Emphysema and chronic bronchitis are the two most common conditions that comprise COPD. The inflammation of the bronchial tubes of the lungs is known as COPD, whereas emphysema is a condition wherein the lungs' alveoli get damaged.
What Are the Signs and Symptoms of Asthma and COPD?
The symptoms of asthma and COPD vary from person to person. However, in most cases, the following symptoms are usually noted:
-
Wheezing.
-
Shortness of breath.
-
Chest pain.
-
Tightness in the chest.
-
Coughing.
-
Recurrent respiratory tract infections.
-
Swelling in the legs, feet, and ankles.
-
Difficulty sleeping.
-
Some people might show weight loss in the later stages of COPD.
Information About Vilanterol for Patients:
Vilanterol mainly combines an inhaled corticosteroid medication (ICS), Fluticasone furoate, and a long-acting beta 2-adrenergic agonist (LABA). ICS, like Fluticasone furoate, reduces the inflammation of the lungs, which can lead to breathing problems. LABA medications like Vilanterol relax the muscles of the airways present around the lungs to prevent the symptoms, including cough, tightness of the chest, wheezing, and shortness of breath. These symptoms usually occur when the muscles around the airways tighten due to mucus deposition, making it difficult for the patients to breathe.
The following important points must be noted about the drug:
-
Vilanterol is not used to relieve sudden breathing problems because it will not replace the rescue inhaler.
-
The drug should not be administered in children and adolescents because no studies have been conducted to assess the safety and efficacy of the drug in children and adolescents below 18 years of age.
Is Vilanterol Effective Against Asthma and COPD?
Vilanterol is effective against asthma and COPD and is used against asthma and COPD in the following manner:
Vilanterol for Asthma:
-
Vilanterol is a prescription medication used once daily as a single inhalation dose. It prevents and controls asthma and wheezing symptoms, making breathing easier.
-
When long-acting beta 2-adrenergic agonists like Vilanterol have been used alone, the risk of hospitalization and death from asthma problems increases. Therefore when an inhaled corticosteroid and a LABA are used together, there is no significant increased risk of hospitalization and death from asthma and COPD problems.
-
Vilanterol is not recommended for patients whose asthma is under control and is taking the medications for asthma. People taking inhaled corticosteroids for asthma management must refrain from using Vilanterol. This drug is only for asthma patients and requires LABA and inhaled corticosteroids.
Vilanterol for COPD:
VIlanterol 100/25 is used to manage COPD, chronic lung disease including emphysema, bronchitis, or both. It is used as a single inhalation dose once daily to improve the symptoms of COPD. So, the patient can breathe properly after taking the drug and reduce the number of flare-ups (worsening of the COPD symptoms).
What Should the Patient Inform the Doctor Before Taking Vilanterol?
Before taking Vilanterol, the patient must inform the doctor if he or she has:
-
Cardiac diseases.
-
High blood pressure (hypertension).
-
Epilepsy (seizures).
-
Thyroid problems.
-
High blood glucose levels or diabetes.
-
Liver diseases.
-
Osteoporosis (reduced bone density).
-
Problems related to the immune system.
-
Eye problems such as glaucoma, cataracts, increased pressure in the eyes, and changes in vision.
-
Viral, bacterial, fungal, or parasitic infection.
-
Is allergic to milk proteins.
-
Is exposed to chickenpox or measles virus.
-
Is pregnant or planning to conceive. However, no studies have been done to determine the effects of Vilanterol on the unborn baby.
-
Is a lactating mother. It has not been known if Vilanterol can pass from the breast milk to the baby and harm him.
-
Takes prescription, over-the-counter drugs, vitamins, and herbal supplements because Vilanterol can interact with certain specific medications and cause serious side effects.
Takes the following medications:
-
Antifungals:
-
Itraconazole.
-
Ketoconazole.
-
Voriconazole.
-
-
Beta-blockers:
-
Atenolol.
-
Labetalol.
-
Metoprolol.
-
Nadolol.
-
Propranolol.
-
-
Diuretics or Water Pills.
-
HIV (Human Immunodeficiency Virus) Protease Inhibitors:
-
Indinavir.
-
Lopinavir.
-
Nelfinavir.
-
Ritonavir.
-
Saquinavir.
-
-
Has stopped taking the following antidepressants over the past two weeks:
-
Amitriptyline.
-
Amoxapine.
-
Clomipramine.
-
Desipramine.
-
Doxepin.
-
-
Is allergic to Vilanterol or the other ingredients of the drug.
-
Has sudden severe symptoms of asthma or COPD.
-
Is planning to undergo dental surgery or any other surgeries. The patient must inform the doctor if he is taking Vilanterol.
How Should the Patient Use Vilanterol?
The patient must read the instructions carefully before taking the medications and keep the following things in mind:
-
The patient must use Vilanterol only by getting proper training from the doctor.
-
The doctor will provide the necessary instructions to the patient on how to use the inhaler for asthma and COPD.
-
As Vilatnerol comes in two different strengths, the doctor will prescribe the strength that is best for the patient.
-
The patient must use the drug as prescribed by the doctor and must not overuse it as it can lead to serious side effects.
-
Use a single inhalation of Vilanterol once a day.
-
Use the drug at the same time daily.
-
The patient must take Vilanterol immediately if he missed the dose.
-
The patient must not take two doses at the same time. He can take the next dose of the drug at the usual time.
-
If a patient has had an overdose of Vilanterol, he must call the doctor immediately and go to the nearby emergency room. The patient might experience unusual symptoms like shortness of breath, chest pain, increased heart rate, and shakiness.
-
Do not use other medications that contain long-acting beta 2-agonist (LABA). The patient can consult the doctor to know which medications contain LABA.
-
Use Vilanterol only after consulting the doctor, and the doctor might change the patient’s medications if required.
-
An important point to be noted is that Vilanterol does not relieve the sudden symptoms of asthma or COPD, so the patient must not take the drug to relieve those symptoms.
-
The patient must keep a rescue inhaler with him to relieve the sudden symptoms of asthma.
The patient must consult the doctor if:
-
His breathing problem worsens.
-
He needs to use the rescue inhaler more than once.
-
The rescue inhaler does not work well in relieving the symptoms.
-
The peak flow meter results decrease.
What Are Some of the Side Effects of Vilanterol?
Vilanterol can cause the following side effects:
Fungal infection in the mouth and throat (oral thrush). The patient must rinse his mouth after inhaling the drug to prevent fungal infection.
People suffering from COPD have higher chances of getting pneumonia. The patient must consult the doctor regarding the following symptoms related to pneumonia:
-
Increased sputum production.
-
Chills.
-
Change in the color of mucus.
-
Fever.
-
Persistent cough.
-
Increased respiratory problems.
-
A weakened immune system increases the chances of acquiring infections (immunosuppression).
-
Adrenal insufficiency is the most common side effect of Vilanterol. It is a condition in which the adrenal glands produce insufficient steroid hormones. This condition usually arises when the patient stops taking oral corticosteroids and takes inhaled corticosteroids like Vilanterol. Adrenal insufficiency symptoms are likely to worsen if the body is under stress due to fever, infections, trauma, or wounds during the transition period.
The symptoms of adrenal insufficiency are listed below:
-
Tiredness.
-
Lack of energy.
-
Nausea.
-
Vomiting.
-
Weakness.
-
Low blood pressure.
Some patients have sudden breathing problems immediately after taking Vilanterol. In such situations, the patient must stop taking the drug and consult the doctor.
Severe allergic reactions might occur, and the patient presents with the following symptoms:
-
Rashes.
-
Hives.
-
Swelling of the face, mouth, and tongue.
-
Breathing problems.
The drug produces different side effects on different organs, which are listed below:
-
Effects on the Heart:
-
Increased blood pressure.
-
Chest pain.
-
Fast or irregular heartbeat.
-
-
Effects on the Central Nervous System:
-
Nervousness.
-
Tremors.
-
Weakness or the thinning of the bones is known as osteoporosis.
Eye problems:
-
Glaucoma.
-
Increased pressure in the eyes.
-
Cataracts.
-
Changes in vision.
-
High blood sugar levels or hyperglycemia can lead to increased thirst, tiredness, and frequent urination.
-
Hypokalemia or low potassium levels.
-
Delayed growth in children.
Some of the common side effects of Vilanterol are listed below:
-
COPD and Asthma:
-
Runny nose.
-
Sore throat.
-
Upper respiratory tract infections.
-
Headaches.
-
Thrust in the mouth and throat.
-
Back pain.
-
Pneumonia.
-
Bronchitis.
-
Inflammation of the sinuses (sinusitis).
-
Cough.
-
Pain in the throat and mouth.
-
Hypertension.
-
Joint pain.
-
Fever.
-
Flu.
-
Hoarseness of the voice.
-
How Should the Patient Store the Drug?
The patient must store the drug in the following ways:
-
Store Vilanterol at room temperatures between 68 to 77 degrees Fahrenheit.
-
Keep the drug in a dry place and away from heat and sunlight.
-
Store Vilanterol in an unopened tray and open only when it is to be used.
-
Throw the drug in the trash six weeks after opening the tray and when the counter reads zero.
-
Keep Vilanterol out of reach of children.
General Information About the Safe and Effective Use of Vilanterol:
Sometimes, the doctor might prescribe the medications for uses other than those listed in the patient instruction leaflet. However, the patient must ensure that he does not use Vilanterol for a condition that was not prescribed. In addition, the patient must not give the drug to other people having similar symptoms because the usage of the drug varies from patient to patient. Only after the doctor examines the patient clinically, Vilanterol can be used; otherwise, it might harm other people. The patient can ask the pharmacist or doctor about the information written for doctors and healthcare professionals.
Information for Doctors:
Description:
Vilanterol 100/25 and 200/25 are inhalation powders for oral inhalation and are used in combination with Fluticasone furoate. One of the active components od Fluticasone furoate is a synthetic trifluorinated corticosteroid having the chemical name 6alpha,11beta,16alpha,17alpha)-6,9-difluoro-17-{[(fluoro-methyl)thio]carbonyl}-11-hydroxy-16-methyl-3-oxoandrosta-1,4-dien-17-yl 2-furancarboxylate.
Fluticasone furoate is a white powder having a molecular weight of 538.6 and the empirical formula of C 27 H29 F3 O6 S. This powder is mostly insoluble in water. Another active component of the drug is Vilanterol trifentate, a white powder with a molecular weight of 774.8. Vilanterol and fluticasone furoate combination is supplied in the inhaler, a light gray or pale blue. It is a light blue inhaler containing two foil blister strips.
Each blister on one strip contains a white powder mixture of micronized Fluticasone furoate (100 or 200 mcg) and lactose monohydrate 12.4 mg. The blister on the other strip contains a white powder mix of Vilanterol terfanatate (40 mcg, equivalent to 25 mcg of Vilanterol) and lactose monohydrate (12.34 mg). The lactose monohydrate mainly contains milk proteins. When the inhaler is activated, the powder in both the blisters gets exposed and is ready for dispersion into the airstream created by the patient when he inhales through the mouthpiece. As per the in vitro study reports, Fluticasone furoate 92 mcg and 184 mcg, and Vilanterol 22 mcg are delivered per blister when tested at the flow rate of 60 L/minute for four seconds.
Clinical Pharmacology:
Mechanism of Action:
As Vilanterol is used in combination with Fluticasone furoate for the management of asthma and COPD, the mechanisms of action of both the drugs have been described separately:
Mechanism of Action of Vilanterol:
Vilanterol belongs to the class of LABA. In vitro studies have shown that the functional selectivity of Vilanterol is similar to Salmeterol. As the study reports, beta 2-receptors are the predominant adrenergic receptors in the smooth muscles around the bronchioles. On the contrary, beta 1-receptors are the predominant receptors in the cardiac muscles.
However, it has been discovered that beta 2-receptors comprise 10 to 50 % of the portion of the total adrenergic receptors of the human heart. The main pharmacologic effect of Vilanterol is that it stimulates the intracellular adenyl cyclase enzyme that converts adenosine triphosphate to cyclic-3′,5′-adenosine monophosphate (cyclic AMP). An elevation in the cyclic AMP levels causes relaxation of the smooth bronchial muscles and inhibition of hypersensitivity mediators from the mast cells.
Mechanism of Action of Fluticasone Furoate:
It is a synthetic tri fluorinated corticosteroid that has anti-inflammatory activity. As per the study reports, Fluticasone furoate’s binding capacity for human glucocorticoid receptors is 30 times more than that of Dexamethasone and two times more than that of Fluticasone propionate. The pharmacological mechanism by which Fluticasone furoate inhibits asthma and COPD symptoms are still unknown. Inflammation is the most important component in the pathogenesis of asthma and COPD. Corticosteroids have a wide range of actions on the different types of cells of the body and inflammatory mediators. As per the study reports, Fluticasone furoate activates the glucocorticoid response element, inhibits the pro-inflammatory factors, and the antigen-induced lung eosinophilia.
Pharmacodynamics:
Cardiac Electrophysiology:
A double-blind, multiple-dose, placebo, and a positive controlled trial were done on 85 individuals to study the drug's effect on QTc interval. It was discovered that the QTc interval was prolonged after the patients inhaled the drug. In addition, a dose-dependent elevation in the heart rate was also observed in these individuals.
Effects on the Hypothalamic-Pituitary-Adrenal Axis:
Healthy Subjects:
No significant changes were observed in the serum or urinary cortisol levels in the healthy individuals who inhaled Fluticasone furoate at repeated doses of 400 mcg. However, a decrease in the serum and urinary cortisol levels was observed when Vilanterol was administered higher than the therapeutic dose.
COPD Patients:
When Vilanterol 25 mcg and Fluticasone furoate 100 or 200 mcg were administered in COPD patients for six months, no significant differences were observed in the 24-hour urinary cortisol levels.
Asthma Patients:
A randomized, double-blind parallel-group trial was done in 185 patients, and no significant differences were noted in the serum and urinary cortisol levels. Fluticasone furoate 100 mcg and Vilanterol 25 mcg was administered in such patients.
Pharmacokinetics:
When Fluticasone furoate (200 mcg) and Vilanterol (25 to 100 mcg) were administered in patients, linear pharmacokinetics was observed. In addition, a steady state plasma concentration was achieved after six days when the drug was administered once daily.
Absorption:
The peak plasma concentration of Vilanterol was observed within ten minutes after the drug was inhaled. The absolute bioavailability of the drug was noted to be 27.3 % after inhalation. The oral bioavailability from the absorbed portion of the drug is less than 2 % because the drug has a high first-pass metabolism. Systemic exposure in people with COPD was 24 % higher than that observed in healthy individuals, whereas, in asthma patients, systemic exposure was lower than 21 %.
Distribution:
When Vilanterol was administered intravenously in healthy individuals, the mean volume of distribution was observed to be 165 L. The binding of Vilanterol to human plasma protein was noted to be 93.9 %.
Metabolism:
The CYP3A4 principally metabolizes Vilanterol to the metabolites, significantly reducing beta one and beta 2-agonist activity.
Elimination:
After Vilanterol was administered orally, it was noted that the drug got eliminated by metabolism, and the metabolites were excreted in the urine and feces. The plasma elimination half-life of Vilanterol after the inhalation at 25 mcg 21.3 hours in people with COPD and 16 hours in people with asthma.
Patients With Hepatic Impairment:
No hepatic impairment was noticed when Fluticasone furoate and Vilanterol 200 mcg/25 mcg were administered repeatedly for seven days. No clinically relevant effects of Fluticasone furoate and Vilanterol were seen on serum potassium levels or heart rate in patients with hepatic impairment. In patients with moderate hepatic impairment receiving Fluticasone furoate/Vilanterol 200 mcg/25 mcg, the serum cortisol levels were reduced by 34 %. Therefore patients with moderate or severe hepatic impairment must be monitored closely.
Indications and Usage of Vilanterol:
Management of Asthma:
Vilanterol is mainly indicated for once-daily treatment of asthma in patients 18 years of age or above. It must be used only for those patients whose asthma cannot be adequately controlled by long-term asthma medications or inhaled corticosteroids. When inhaled corticosteroids and LABA manage asthma, the Vilanterol and Fluticasone furoate combination is a perfect choice.
Management of COPD:
Vilanterol 100/25 is indicated for the long-term daily maintenance treatment of airflow obstruction in patients with COPD, including emphysema and chronic bronchitis. In addition, the drug also works to reduce exacerbations in patients with a history of COPD.
Important Note:
Vilanterol is not indicated for the management of acute bronchospasm.
Dosage Forms and Strengths:
Inhalation Powder:
It is available as a disposable light gray and a pale blue plastic inhaler containing two foil blister strips of powder for inhalation purposes only. One strip contains Fluticasone furoate, whereas the other strip contains Vilanterol.
Contraindications:
Vilanterol must not be used under the following circumstances:
- Primary management of status asthmaticus or other episodes of acute COPD or asthma where indeed extensive management measures are required.
- Severe hypersensitivity to milk proteins, Fluticasone furoate, Vilanterol, or other constituents.
Drug Warnings and Precautions:
Serious Asthma-Related Events - If Vilanterol is used alone and not in combination with Fluticasone furoate, the risk of asthma-related deaths increases. As per the trial reports, it has been noted that using LABA as monotherapy increases the risk of asthma-related hospitalization in adolescent and pediatric patients.
However, when Vilanterol is used with Fluticasone furoate, no significant increase in the risk of severe asthma-related events was noted. Four large randomized, double-blind placebo-controlled trials were done for 26 weeks to assess the risk of serious asthma-related events when LABA was used in combination with inhaled corticosteroids. It was found in the trial results that the patients who took the combination did not suffer from serious side effects or asthma-related complications.
Acute Episodes of Disease - Vilanterol should not be administered in patients with rapidly deteriorating or potentially life-threatening episodes of asthma or COPD. People suffering from COPD might experience acute symptoms over several days or longer. If Vilanterol 100/25 mcg fails to control asthma or COPD symptoms, the patient requires more short-acting beta 2-agonist than usual.
So, Vilanterol 100/25 is inappropriate for the patient, and the COPD treatment regimen must be reconsidered. Similarly, Vilanterol must not be used for managing acute symptoms or as rescue therapy for treating acute episodes of bronchospasm.
Instead, acute symptoms must be managed with inhaled short-acting beta2-agonists. Before taking Vilanterol, patients must be asked to discontinue the other oral or inhaled, short-acting beta2-agonists regularly. These drugs must be used only for symptomatic relief of acute respiratory symptoms.
Excessive Use of Vilanterol and Use With Other Drugs - Vilanterol should not be used at a higher dose than recommended or in conjunction with other medications containing LABA. This is because clinically significant effects on the cardiovascular system have been noted after the patients took Vilanterol with other sympathomimetic drugs.
Effects of Inhaled Corticosteroids - During the clinical trials, it was noted that the patients treated with Vilanterol developed localized infections of the mouth and pharynx with Candida albicans. When such an infection occurs, appropriate local or systemic antifungal therapy must be used. The patient must rinse his mouth with water after inhaling the drug to prevent infections.
Pneumonia - An increased incidence of pneumonia was reported in patients with COPD receiving Vilanterol. Some patients were hospitalized because the severity of pneumonia increased to a large extent. The doctors must remain vigilant about the fatal effects of the drug on people suffering from pneumonia.
Immunosuppression - People using drugs that suppress the immune system are more vulnerable to acquiring infectious diseases. Vilanterol should be used carefully in adults diagnosed with chicken pox and measles. Instead, these individuals should be vaccinated before the administration of the drug.
Paradoxical Bronchospasm - Vilanterol can produce paradoxical bronchospasm if used with other medications, which could be life-threatening. If the patient suffers from paradoxical bronchospasm after taking Vilanterol, he must be immediately treated with a short-acting bronchodilator.
Hypersensitivity Reactions - Hypersensitivity reactions including angioedema, rashes, and urticaria might occur after inhaling Vilanterol. Hence, the drug must be discontinued if such reactions occur, especially in patients with allergies to milk proteins.
Cardiovascular Effects - Vilanterol should be immediately discontinued if it produces the following clinically significant cardiovascular effects:
- Increased pulse rate.
- Supraventricular tachycardia.
- Extrasystoles.
- Cardiac arrhythmias.
- Flattening of the T wave.
- Prolongation of the QTc interval.
- Depression of the ST segment.
Reduction in Bone Density - Reduction in bone density was observed in patients who have been on long-term administration of Vilanterol. Therefore, patients with a family history of osteoporosis, tobacco use, advanced age, and who are using the drugs that reduce bone mass must be carefully monitored for the side effects of Vilanterol.
Cataracts and Glaucoma - Cataracts and glaucoma or increased intraocular pressure have been reported in people with COPD and asthma following the long-term administration of Vilanterol. Such patients must be referred to an ophthalmologist.
Hyperglycemia and Hypokalemia - Vilanterol has been known to elevate blood glucose levels and lower blood potassium levels in diabetic patients.
What Are the Adverse Reactions of Vilanterol?
The adverse reactions of Vilanterol are listed below:
- Serious asthma-related events.
- Candida albicans infection.
- Increased risk of pneumonia.
- Immunosuppression.
- Hypercorticism.
- Adrenal suppression.
- Osteoporosis.
Postmarketing Experience:
Some other adverse reactions were noted from a small group of the population after the drug was launched in the market. However, these reactions cannot be relied upon because they were noted in a few individuals.
The following adverse reactions were seen:
- Cardiac Disorders - Palpitations, tachycardia.
- Disorders of the Immune System - Anaphylaxis, angioedema, rashes, and urticaria.
- Metabolic Disorders - Hyperglycemia.
- Musculoskeletal Disorders - Muscle spasms.
- Nervous System Disorders - Tremors.
- Psychiatric Disorders - Nervousness.
- Respiratory and Mediastinal Disorders - Paradoxical bronchospasm.
Drug Interactions:
Vilanterol must be carefully used or avoided in patients taking the following group of drugs:
- Inhibitors of Cytochrome P450 3 A4 - It includes the following drugs:
- Ritonavir.
- Clarithromycin.
- Conivaptan.
- Indinavir.
- Itraconazole.
- Lopinavir.
- Nefazodone.
- Troleandomycin.
- Voriconazole.
- Monoamine oxidase inhibitors and Tricyclic antidepressants - It includes the following drugs:
- Monoamine Oxidase Inhibitors:
- Isocarboxazid.
- Phenelzine.
- Selegiline.
- Rasagiline.
- Monoamine Oxidase Inhibitors:
2. Tricyclic Antidepressants:
-
-
- Amitriptyline.
- Clomipramine.
- Doxepin.
- Imipramine.
- Nortriptyline.
- Protriptyline.
-
Beta-Adrenergic Receptor Blockers - The following drugs cause severe bronchospasm if administered with Vilanterol:
-
- Carvedilol.
- Labetalol.
- Nadolol.
- Propranolol.
Non-potassium Sparing Diuretics - Hypokalemia occurs if Vilanterol is administered with the following drugs:
-
- Chlorothiazide.
- Hydrochlorothiazide.
- Bumetanide.
- Furosemide.
- Torsemide.
Non-clinical Toxicology:
Carcinogenesis, Mutagenesis, and Impaired Fertility:
No studies were done to evaluate the carcinogenesis, mutagenesis, and impairment of fertility of Vilanterol and Fluticasone furoate combination. However, animal studies had revealed that Vilanterol caused slight increases in carcinogenesis when they were administered at 29,500 mcg/kg/day. Vilanterol did not show any mutagenic potential when a genotoxicity assay was done, and no impairment of fertility was noted in animal studies.
Instructions for Drug Use (Vilanterol and Fluticasone Furoate Inhalation Powder):
The patient should keep the following instructions in mind before taking the drug:
- If the patient opens or closes the cover without inhaling the medication, he might lose the dose.
- The lost dose will be present inside the inhaler, but it will not be available for use.
- Do not take a double dose or extra dose with one inhalation.
How Should the Inhaler Be Used?
- Vilanterol comes in a tray.
- Peel the lid backward to open the tray of the drug.
- The tray consists of a desiccant that helps reduce moisture.
- Do not eat or inhale the tray cover.
- Throw away the lid in the household trash.
Important Points to Be Noted About Vilanterol:
- The inhaler mainly contains 30 doses. It might contain 14 doses if a sample or an institutional pack is used).
- The patient will hear a clicking sound every time he opens the inhaler, and the dose is ready to be inhaled. A decrease in the number over the counter will be visible.
- If the patient opens or closes the cover without inhaling the medicine, he might lose the dose.
- Avoid opening the inhaler cover unless it is ready to be used. After the inhaler is ready, do not close the cover until the medicine has been inhaled to prevent the wastage of the drug.
- Specify the “tray open” and “discard” date on the inhaler label. The discard date is usually six weeks after the inhaler is used.
- Before the patient uses the inhaler for the first time, the counter should show the number 30, which indicates the number of doses in the inhaler.
- The patient must prepare one dose of the medicine each time he opens the cover.
- The counter counts down by one each time the cover is opened.
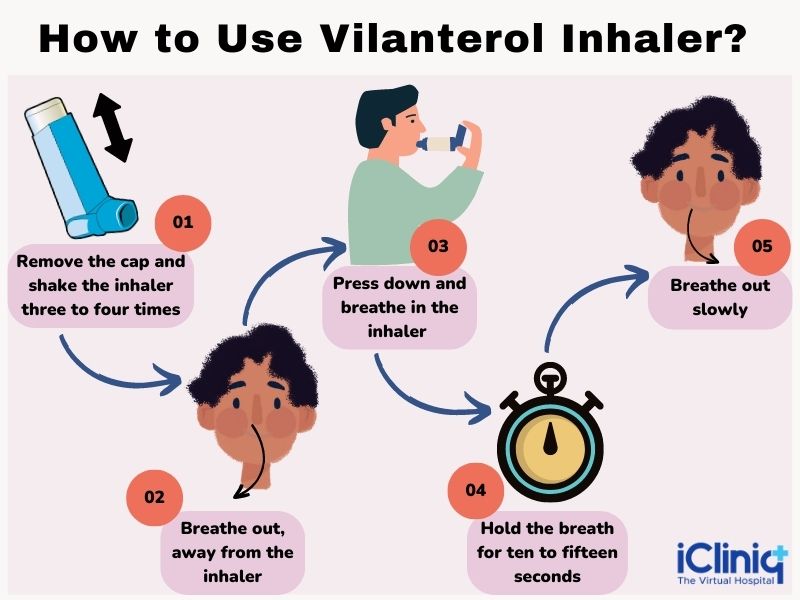
Steps of Using the Inhaler:
Step 1 - Open the Cover of the Inhaler:
- Slide the cover present on the inhaler downwards to expose the mouthpiece. The patient will hear a click sound and must shake the inhaler before use.
- The inhaler can be used now. However, the inhaler will not deliver the medication if the counter does not count down as the clicking sound is heard.
Step 2 - Breathe Out:
- The patient must hold the inhaler at a distance from the mouth and exhale or breathe out completely. Do not breathe out in the mouthpiece.
Step 3 - Inhale the Medicine:
- Put the mouthpiece between the lips and close the lips firmly around it. Make sure the patient’s lips cover firmly over the curved shape of the mouthpiece.
- Take a long, deep, and steady breath through the mouth, but do not breathe through the nose.
- Do not block the air vent with your fingers.
- Remove the inhaler from the mouth and hold your breath for three to four seconds.
Step 4 - Exhale Slowly and Gently:
- The patient cannot taste or feel the medication using the inhaler correctly.
- Avoid taking another dose from the inhaler to taste or feel the medication.
Step 5 - Close the Inhaler:
- Clean the mouthpiece if required using dry tissue before closing the cover.
- Slide the cover-up over the mouthpiece as far as it goes.
Step 6 - Rinse Your Mouth:
- The patient must rinse his mouth with water and spit it immediately after using the inhaler. Do not swallow the water.
- When less than ten doses remain in the inhaler, the left half of the counter shows red. It is a reminder that the patient must get a refill.
- After the patient has inhaled, the counter reads zero.
- Throw the empty inhaler in the household trash.
How Is Vilanterol Supplied?
Vilanterol is supplied as a disposable light gray and a pale blue plastic inhaler containing two foil strips. Each foil strip has 30 blisters containing Fluticasone furoate (100 or 200 mcg) and Vilanterol (25 mcg per blister). One blister from each strip is used to create one dose. The inhaler is packed in a moisture-protected foil tray with a desiccant and a peelable lid. The drug must be stored at room temperatures between 68 to 77 degrees Fahrenheit. The drug must be removed from the tray only when it has to be used. Discard the drug six weeks after use as the inhaler is not reusable.
Overdosage:
No data is available related to the overdosage of the drug. However, Vilanterol is used in combination with Fluticasone furoate, so the signs and symptoms might arise due to the overdosage of Vilanterol alone.
The following symptoms are noted after the overdosage of Vilanterol:
- Seizures.
- Angina.
- Hypertension.
- Hypotension.
- Tachycardia.
- Arrhythmias.
- Nervousness.
- Headache.
- Tremor.
- Muscle cramps.
- Dry mouth.
- Palpitations.
- Nausea.
- Malaise.
- Fatigue.
- Insomnia.
- Hyperglycemia.
- Hypokalemia.
- Metabolic acidosis.
Some More Facts About Vilanterol:
Use in Specific Populations:
Pregnancy:
Insufficient data is available regarding the use of Vilanterol in pregnant females. However, when Vilanterol and Fluticasone furoate was administered in combination in pregnant females, no significant abnormalities were detected in the fetal structure. Nevertheless, the risk of pre-eclampsia and low birth weight babies increases in females with poor or moderately controlled asthma. Therefore, pregnant females must be carefully monitored for the side effects, and the medication dosage must be adjusted accordingly.
Lactation:
There is no information available regarding the presence of Vilanterol in human milk, the effects on the breastfed child, or milk production. However, low concentrations of other inhaled corticosteroids have been detected in human milk. Therefore, the health benefits of breastfeeding must be measured and monitored for the lactating mothers receiving Vilanterol.
Pediatric Population:
Vilanterol must not be administered to children and adolescents below 18 years. A randomized, double-blind placebo-controlled clinical trial has demonstrated that orally inhaled corticosteroids reduce the growth velocity when given to children and adolescents.
Geriatric Use:
A clinical trial was done on 4820 patients 65 years or above suffering from COPD to evaluate the safety and effectiveness of Vilanterol. No overall clinically significant differences in the safety and effectiveness of the drug were noted in these patients.
Clinical Trial:
COPD:
Four confirmatory randomized, placebo-controlled, and double-blind trials were done on more than 24,000 patients for six to 12 months to evaluate the safety and efficacy of Vilanterol. The patients were randomized to Vilanterol 100/25, 200/25, Fluticasone furoate 100 mcg, 200 mcg, and placebo during the first trial. In the second trial, the patients were randomized to Vilanterol 100/25, Fluticasone furoate/Vilanterol 50 mcg/25 mcg, and Fluticasone furoate 100 mcg, and placebo.
Trial Results:
The efficacy of Vilanterol was measured on the basis of weighted mean FEV (forced expiratory volume), and it was found that Vilanterol 100/25 was more effective than placebo and Fluticasone furoate.
Asthma:
The safety and efficacy of Vilanterol for asthmatic patients were evaluated based on the four double-blind, randomized, and placebo-controlled trials on 9969 patients. In the first trial, the patients were randomized to Vilanterol 100/25 and Fluticasone furoate 100 mcg or placebo. During the second trial, the patients were randomized to Vilanterol 100/25, 200/25, Fluticasone furoate 100 mcg, 200 mcg, and placebo.
Trial Results:
The trial results indicated clinically significant lung function improvements in patients receiving Vilanterol 100/25 or 200/25 over the 24 hours. Also, a 25 % reduction in the asthma exacerbation rate was noted.













