Drug Overview
Fenfluramine is a derivative of amphetamines and a selective serotonin reuptake inhibitor (SSRI). It causes inhibition of the uptake and release of serotonin. Fenfluramine is used for the management of seizures in children (two years of age and older) with Dravet syndrome. Dravet syndrome is an uncommon type of epilepsy seen in children during the first year of life. It is associated with different signs and symptoms that cause developmental delays and loss of coordination in children. Fenfluramine is used to control seizures in children with Dravet syndrome and was approved in 1973 by the FDA (Food and Drug Administration) for the management of seizures. Read the article to learn more about the uses, side effects, and pharmacology of the drug Fenfluramine.
How Does the Drug Fenfluramine Work?
The drug Fenfluramine is an anticonvulsant drug that works by binding to a serotonin reuptake pump. It inhibits the serotonin receptors uptake and release, thereby causing an increase in the number of serotonin receptors, which in turn reduces seizure activity.
Uses
Fenfluramine is an accepted drug for the control of seizures in children aged two years or older with:
-
Dravet Syndrome: It is associated with seizures that develop in children during the first year of life. It results in loss of coordination and developmental delay.
-
Lennox-Gastaut Syndrome: It is associated with seizures and delay in child development.
Doses
-
Fenfluramine is available in the form of oral solution at the dose of 2.2 mg per mL.
-
It is usually advised to consume two times a day with or without food.
For Patients
What is Dravet Syndrome?
Dravet syndrome is a genetic disorder characterized by seizures and loss of balance or coordination in children during the first year of life. It occurs due to a mutation of the SCN1A (sodium voltage-gated channel alpha subunit 1) gene. Other associated symptoms are behavioral problems, sleep disturbances, and severe seizures. The seizures are usually prolonged jerky movements, mostly affecting one side of the body. There are chances of SUDEP (sudden unexplained death in epilepsy) in many patients with Dravet syndrome. Childrens with Dravet syndrome usually develop epileptic episodes four times a month for around ten minutes continuously.
Learn More About Fenfluramine
When and Why to Take Fenfluramine Medication?
Fenfluramine is usually prescribed by the doctor for controlling seizures in children of ages two or older after a thorough examination of the patient. Fenfluramine is an accepted drug by the FDA (Food and Drug Administration) for the control of seizures in children with Dravet syndrome. Fenfluramine is available in oral solution form at a dose of 2.2 mg per mL of solution. It should be taken on an exact schedule as prescribed by the doctor.
How Effective Is Fenfluramine Against Seizures?
Fenfluramine helps control the attack of seizures. It does not cure seizures. It is used in children of two years of age and older. Fenfluramine works by inhibiting serotonin uptake and release.
Things to Inform the Doctor Before They Prescribe Fenfluramine
-
Inform the doctor in case of any allergy to any drug or its composition.
-
In case of any systemic disease like diabetes, hypertension, eye disease, cardiovascular disorders, and renal disease, make sure to inform the doctor prior.
-
Inform the doctor if planning a pregnancy, or pregnant and breastfeeding status.
-
Inform the doctor if under any medications in the past or any current ongoing medications.
Starting Fenfluramine Drug
How to Take Fenfluramine?
-
The oral suspension form is available for fenfluramine.
-
It should be consumed in exact doses and frequency as recommended by the doctor.
-
It is usually taken orally two times a day with or without food.
-
The dose prescribed by the doctor can be measured using a sterile syringe and the measured amount of solution can be taken by spoon. The syringe should be washed properly with tap water and can be reused. Avoid measuring the dose from the spoon as it may result in the alteration of the dose prescribed.
Things to Do After Taking Fenfluramine
-
Observe for improvement in the symptoms and update the doctor in the follow-up visits.
-
In case of worsening symptoms, inform the doctor immediately.
-
In case of any adverse reactions developed in response to the drug, it is mandatory to inform the doctor.
-
Do not stop the drug abruptly, Always inform the doctor before stopping the drug.
Look Out for the Side Effects
1. Common Side Effects:
-
Vomiting.
-
Loose motions.
-
Fever.
-
Insomnia (difficulty in falling asleep).
-
Cough.
-
Unsteadiness.
-
Drooling of saliva.
-
Stomach upset.
-
Weight loss.
-
Loss of appetite.
-
Dizziness.
-
Ear drainage.
-
Voice changes.
-
Tremor.
2. Serious Side Effects:
-
Shortness of breath.
-
Chest pain.
-
Tiredness or weakness.
-
Increased heart rate.
-
Swelling in the feet or ankles.
-
Irregular pulse.
-
Lightheadedness.
-
Bluish discoloration of the skin or lips.
In case any of the above serious side effects are observed, like breathlessness, chest pain, or an increased heart rate, call the medical emergency line immediately and stop taking the medications.
Dietary Alterations
The drug Fenfluramine can be taken with or without food, as it does not react with food. No diet changes are needed when on Fenfluramine medication unless suggested by the doctor.
What Should Be Done If Forgot to Take a Dose?
If the dose is missed, take the medication as soon as possible. If it is time for the next dose, then skip the missed dose and continue with the normal dosing schedule.
What Should Be Done in the Case of Overdose?
An overdose of the drug Fenfluramine can lead to muscle spasms, respiratory depression, tachycardia, tremors, etc. In case of overdose, contact the poison control helpline number immediately.
Storage of Fenfluramine
-
The drug should be stored in an airtight container.
-
It should not be frozen or refrigerated.
-
The drug should be stored at room temperature, away from moisture.
-
Unused drug bottles that have expired and have crossed three months after opening should be discarded.
Avoid Self-Prescription
The drug Fenfluramine is only available on prescription. The drug is prescribed by the doctor after a thorough examination of clinical signs and symptoms. The drug Fenfluramine should be consumed according to the exact dose and schedule prescribed by the doctor.
Staying On Fenfluramine
For Doctors
Indications
The drug Fenfluramine is indicated in:
-
Children of two years or above with Dravet syndrome to control seizures.
-
In children with Lennox-Gastaut Syndrome for control of seizure attacks.
Pharmacology
Mechanism of Action
The drug Fenfluramine helps control seizures due to Dravet syndrome by inhibiting the release and uptake of serotonin. It increases levels of serotonin in the brain (extracellularly) by reversing serotonin transport. Fenfluramine usually binds to Sigma 1 receptors and alters its activity.
Pharmacodynamics
The drug Fenfluramine increases the levels of serotonin extracellularly. Fenfluramine works on serotonin receptors and alpha-one receptor antagonists, resulting in decreased epileptic attacks.
Chemical Taxonomy:
Kingdom- Organic compounds.
Superclass- Benzenoids.
Class- Benzene and subsituted derivatives.
Subclass- Phenethylamines.
Direct Parent- Salicylic acids.
Ingredients
Active Ingredient: Fenfluramine hydrochloride is the only active ingredient present in the drug.
Inactive Ingredients:
-
Glucose.
-
Sodium ethyl parahydroxybenzoate.
-
Sodium methyl parahydroxybenzoate.
-
Sulfur dioxide.
Doses and Forms
-
The drug Fenfluramine is available in oral solution form at the dose of 2.2 mg per mL of solution. It is prescribed by the doctor twice a day with or without food.
-
The drug Fenfluramine should be consumed as per the directions by the doctor.
-
Usually, the drug is started with a low dose initially by the doctor and slowly the dose is increased once a week.
-
It is a clear, viscous, and colorless liquid with pH 5.
-
The initial dose of the drug Fenfluramine is determined by the doctor based on the child's weight. The dose is later increased slowly.
-
The drug Fenfluramine is never stopped abruptly. Doctors usually reduce the dose gradually.
Pharmacokinetics
Absorption: The steady-state concentration is around 68 nanograms per milliliter. The bioavailability is around sixty percent.
Distribution: The drug Fenfluramine has an average volume of distribution of 11 liters per kilogram.
Metabolism: The drug Fenfluramine is metabolized in the liver by CYP1A2, CYP2B6, CYP2D6, and CYP3A4 to produce Norfenfluramine (which is an active metabolite) and other metabolites that are in an inactive form.
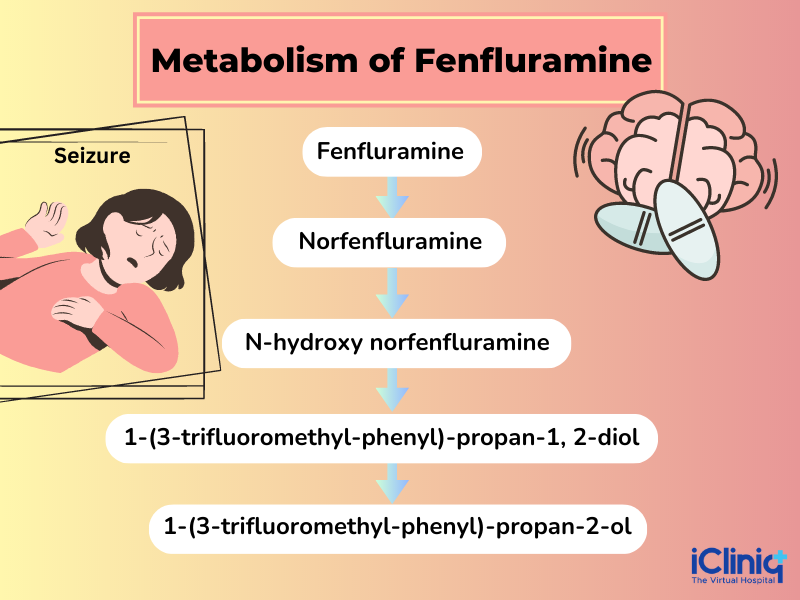
Excretion: Almost ninety percent of the drug Fenfluramine is excreted through urine, and the rest (trace amount) of around five percent is excreted through feces.
Half-Life: The average half-life of Fenfluramine is around 20 hours in a healthy individual.
Plasma Clearance: The average plasma clearance of Fenfluramine is around 24 liters per hour.
Protein Binding
Irrespective of the drug concentration in plasma, the drug Fenfluramine is around fifty percent bound to the plasma proteins.
Bioavailability: The absolute bioavailability of the drug is around 65 to 80 percent.
Toxicity
An overdose of the drug Fenfluramine can result in twitching, tremors, an increased heart rate, mydriasis (dilation of the pupil of the eyes), increased muscle tone, and respiratory failure. It is advised to contact the poison control center immediately in the event of an overdose.
Warning and Precautions
-
Fatal Drug Reactions: It is advised to avoid the medication in patients on monoamine oxidase (MAO) drugs, methylene blue injection, and Selegiline for the past 14 days as it can result in fatal drug reactions. The drug Fenfluramine is advised by the doctor only after 14 days of stopping these drugs.
-
Risk Of Valvular Heart Disease: The drug Fenfluramine carries a risk of valvular heart diseases so it should be avoided in patients with cardiovascular disorders. The doctor usually performs an echocardiogram (ECG) before starting Fenfluramine. An echocardiogram test is repeated every six months for patients during the treatment under Fenfluramine and six months after the final dose of the medications.
-
Risk of Sudden Weight Loss: The drug Fenfluramine can result in a sudden decrease in appetite resulting in the weight loss of a child.
Drug Interactions
-
The risk of adverse events increases when the drug Fenfluramine is given in combination with Benzodiazepines, Acetophenazine, and Acetazolamide.
-
The concentration of Fenfluramine in the serum is increased when the drug is given in combination with Abametapir and Abiraterone. It results in toxicity due to an increase in the serum concentration of the drug.
-
The metabolism of the drug Acebutolol and Acetaminophen is reduced when given in combination with the drug Fenfluramine.
-
Fenfluramine can cause acute confusion if given in combination with monoamine oxidase inhibitors.
Contraindications
-
Fenfluramine should be avoided in patients under monoamine oxidase (MAO) drugs within the past 14 days.
-
Fenfluramine should be avoided in patients under methylene blue and Selegiline for the past 14 days.
-
Fenfluramine should be avoided in patients with cardiovascular diseases like arrhythmias or hypertension.
-
Fenfluramine is contraindicated in patients with glaucoma.
Other Specifications:
-
Fenfluramine in Pregnant and Lactating Women: The safety of the drug Fenfluramine for use in pregnant and lactating women has not been yet reported.
-
Fenfluramine in Pediatric Patients: Further research is still needed to check for the effectiveness and safety of the use of the drug Fenfluramine in children below two years old and in obese children. The drug Fenfluramine can be used effectively in children above two years of age.
-
Fenfluramine in Geriatric Patients: The safety and efficacy of the drug Fenfluramine in elderly patients are yet to be determined.
What is the Risk Evaluation and Mitigation Strategies (REMS) Program?
REMS (Risk Evaluation and Mitigation Strategies) is a program for drug safety by the FDA (Food and Drug Administration). This program is for evaluating the risk of certain medications like Fenfluramine because of their potential side effects. The program mainly concerns the risk-benefit ratio so that the medications outweigh the risk. As the drug Fenfluramine carries a potential risk, it is mostly distributed through this program.
What Are the Clinical Trials Ongoing for the Drug Fenfluramine?
A double-blinded trial is ongoing by the researchers to study the role of Fenfluramine in the cognitive functions of the child. Furthermore, a trial is ongoing to study the effectiveness and safety of the drug Fenfluramine in obese children and children under the age of two. Further research is needed for improved treatment strategies for Dravet syndrome.












