Overview:
Risdiplam is an mRNA splicing modifier. Risdiplam was approved by the FDA (Food and Drug Administration) in 2020 for spinal muscular atrophy treatment. It is used for spinal muscular atrophy because it improves and increases the SMN (survival of motor neuron) gene efficiency by transcription. It provides cheaper and easier oral bioavailability than other alternative spinal muscular therapies.
What Are the Usage and Indications for Risdiplam?
The indication of Risdiplam is for the treatment of spinal muscular atrophy in patients above two months and older.
What Is the Contraindication For Risdiplam?
There are, as such, no contraindications to Risdiplam.
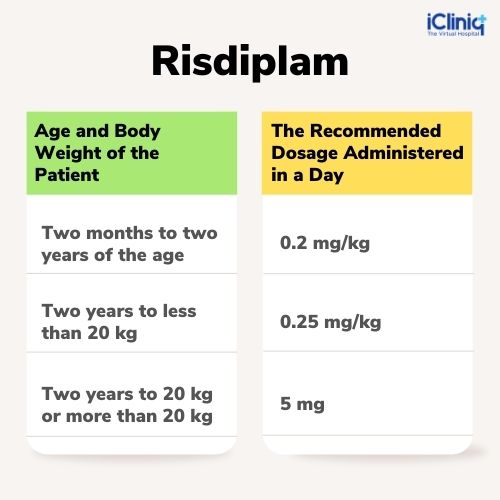
Dosage:
Dosage Form: Oral solution.
Dosage Strength: 60 mg of Risdiplam in 0.75 mg/mL solution.
Administration:
Risdiplam is administered once a time in a day after a meal.
What Are the Adverse Reactions to Risdiplam?
Adverse reactions of Risdiplam are as follows:
- Fever.
-
Diarrhea.
-
Rash.
-
Vomiting.
-
Upper respiratory tract infections.
-
Constipation.
-
Pneumonia.
Special Considerations:
-
Pregnancy: There is no specific data that provides information regarding the safety or risks of Risdiplam in pregnant women. But the studies on animals have presented the adverse effects of developmental malformations like low birth weight babies.
-
Lactation: There is no prevalent data present that provides the effects of Risdaplam on human breast milk. However, the studies in animals have provided information regarding excreted Risdiplam in the breast milk of rats.
-
Females and Males of Reproductive Potential: The studies performed on rats and other animals like monkeys have reported adverse effects which are present in reproductive organs. A pregnancy test for females before starting with Risdiplam is recommended. Risdiplam is not recommended for pregnant women as it can cause embryo-fetal harm. So, it is suggested that female patients have contraception while having Risdiplam for at least one month after the last use of Risdiplam. Using Risdiplam can also harm male fertility as it compromises male patients.
-
Pediatric Use: The safe use of Risdiplam with effectiveness has been established in children at the age of two or more. But the safety and effectiveness in child patients below two months of age have not been estimated.
-
Geriatric Use: No relevant studies are present to provide the safety and effectiveness of Risdiplam use in patients above 65 years of age.
-
Hepatic Impairments: The use of Risdiplam in patients with hepatic impairments with safety and effectiveness has not been established. Risdiplam is metabolized in the liver so that any hepatic impairments can increase the dose present in the body. So, it is not used in hepatic-impaired patients.
For Patients:
What Is Spinal Muscular Atrophy?
Spinal muscular atrophy is a disorder that causes muscle weakness due to motor nerves getting affected. It is mainly caused when there is a lack of specific protein (SMN) because of a defect in genes. It is of three types SMA type I, SMA type II, and SMA type III. Type III is a less severe form than type I, which is also called Werdnig-Hoffman disease.
What Is the Use of Risdiplam Medicine?
Risdiplam is used to treat patients suffering from spinal muscular atrophy, and it is used in only patients who are two years old and above, from the child to adult age group.
How Is Risdiplam Given to the Patient?
Risdiplam is available in liquid form that needs to be prepared. The patient takes medicine after the meal through the mouth or feeding tube.
Taking the Risdaiplam medicine:
-
The dosage prepared is based on the age and weight of the patient for adults. It is five mg once daily.
-
The medicine is not mixed with formula milk or any other milk or liquid and even food. The infants who require these medications are given the medicine after breastfeeding once daily and take water after medicine to ensure that the medicine is swallowed.
-
If the patients are unable to take medicine by mouth, then feeding tubes are used; they need to take medicine by flushing it with water.
-
If the medicine is taken through a syringe, use the prepared syringe for five minutes; if not, throw the prepared medicine in the syringe.
-
The patient should not stop taking medicine on their own; consulting a doctor before stopping is important.
What Are the Precautions Taken While Taking Medicine?
Precautions are:
-
The patient should inform the doctor if the patient is suffering from any liver problems.
-
The patient should inform the doctor about the pregnancy or if the patient is planning it.
-
The patient should talk to the doctor about the birth control methods while being on treatment and take birth control at least one month after the last use of the medicine Risdiplam.
-
The patient should inform the doctor about breastfeeding if the patient does it.
-
The medicine should not be taken with the help of a household spoon.
-
The prepared medicine should be taken within five minutes; otherwise, throw the medicine and use the new medicine.
-
Avoid the medicine being in contact with the skin and eyes.
-
If the patient vomits after taking medicine, then the patient should avoid another dose simultaneously.
-
The patient should inform the doctor if the condition gets worse after using the medicine.
How Is Risdiplam Stored and Discarded?
Risdiplam is stored in refrigerators between 36 to 46 degrees Fahrenheit. But avoid keeping it freezing. And the bottle is kept in the upright position in amber bottles to protect it from sunlight. The unused medicine is discarded after 64 days of preparation. The unused medicine should not be discarded as such. They are discarded as instructed by the Food and Drug Administration. Avoid the medicine from children.
What if the Medicine Got Missed?
If the patient misses the regular dose and remembers to take it within six hours, then the patient should take it within six hours. Or if the patient misses the dose with time more than six hours, then skip the missed dose and take it at regular times the next day.
What Are the Side Effects of Risdisplam?
Side effects are:
-
Fever.
-
Diarrhea.
-
Rash.
-
Lung infection.
-
Runny rose.
-
Sneezing.
-
Cough.
-
Vomiting.
For Doctors:
Risdiplam:
Risdiplam is motor neuron 2 (SMN2), an mRNA splicing modifier. It has a chemical name 7-(4,7-diazaspiro[2.5]octan-7-yl)-2-(2,8 dimethylimidazole [1,2-b] pyridazin-6-yl) pyrido-4H-[1,2-a] pyrimidin-4-one. It has a molecular weight of 401.46 g/mol. It has a molecular formula of C22H23N7O. It has inactive ingredients like ascorbic acid, disodium edetate dihydrate, isomalt, mannitol, polyethylene glycol 6000, sodium benzoate, strawberry flavor, sucralose, and tartaric acid, and an active ingredient Risdiplam.
Dosage and Administration:
Dose Strength: 0.75 mg/mL as 60 mg oral solution, which can be light yellow to yellow to grayish yellow to greenish yellow to light green powder, and after constitution, 80 mL Risdiplam which is greenish yellow to yellow.
Dose Preparation:
The dose preparation is done according to instructions from the healthcare caregiver. It is instructed to prepare a dose with the help of a reusable oral syringe. And Risdiplam is used when drawn. If used in less than five minutes, then prepare a new one.
Dose Administration:
It is taken orally once a day after meals at the regularly scheduled time. The breastfed patients or infants are delivered Risdiplam without mixing it with any milk. And drinking water after the medicine is important. If the patient is on a feeding tube, then Risdiplam is also delivered through the tube.
Preparation of Oral Solution:
The pharmacist prepares Risdiplam powder before delivering it to the patient. The preparation of 0.75 mg/mL oral solution. The powder is handled cautiously and avoids contact with the powder by eyes or skin. If contact occurs, wash the skin with soap and water, and the eyes are to be rinsed with water. The use of disposable gloves is done to avoid contact. Preparation:
-
A gentle tap at the bottom of the glass bottle is done to lose the powder for use.
-
Removal of the cap is done, but avoid throwing it.
-
Pour 79 mL of purified water into the Risdiplam bottle to form a 0.75 mg/mL oral solution.
-
Press the bottle adapter by pushing down the bottle lip.
-
Close the bottle with the cap tightly, shake for 15 seconds, and wait to form a clear solution. If no clear solution forms, then shake it again after 10 minutes.
-
After evaluation, write the expiry date (64 days after it formed).
-
Keep the constituted bottle in the back in amber bottles away from light. The bottle is stored in the refrigerator at two to eight degrees Celsius. Keep the bottle in an upright position with a closed cap.
-
Then select the oral syringes of 6 mL or 12 mL per the patient's dosage requirement.
-
Further, dispense the medicine for use.
What Is the Clinical Pharmacology of Risdiplam?
As spinal muscular atrophy shows chromosome 5q mutation that causes protein deficiency, Risdiplam has shown exon seven inclusion in SMN 2 messenger mRNA transcription and production of SMN protein as the Risdiplam can cause the splicing of genes like FOXM1 and MADD (MAP Kinase Activation Death Domain), which helps in cell cycle regulation.
Pharmacodynamics:
Risdiplam increases the level of SMN protein within four weeks of treatment, and the increase was maintained throughout the treatment period in all SMA types.
Pharmacokinetics:
Absorption: Risdiplam is taken orally after morning meals or after breastfeeding and takes one to four hours to reach maximum plasma concentration.
Distribution: The volume of distribution reaches 6.3 L/kg. Risdiplam binds to serum albumin with no binding to alpha-1 acid glycoproteins.
Elimination: The clearance rate of Risdiplam is 2.1 L/hour, and the half-life of the Risdiplam is 50 hours.
Metabolism: It is metabolized by flavin monooxygenase 1 and 3 and also by CYP3A4, CYP3A7, CYP1A1, and CYP2J2.Risdiplam is a major component in plasma.
Excretion: It is excreted in feces and urine. 53 percent dose is excreted in the feces, and 28 percent is excreted in the urine.
Nonclinical Toxicology:
Carcinogenesis: The carcinogenic evaluation of Risdiplam on humans is not clearly determined, but studies on animals like mice have evaluated that it is not carcinogenic.
Mutagenesis: There are no relevant studies on humans that provide any data regarding the mutation effect of Risdiplam, but studies on animals have shown clastogenic effects.
Impairment of Fertility: The studies done on animals have shown effects on males as their testis were facing atrophy and spermatocytes, but no evaluation on humans is present.
Clinical Studies:
The efficacy of Risdiplam with infantile-onset and later-onset spinal muscular atrophy was determined in two clinical studies.
-
Infantile-Onset Spinal Muscular Atrophy: It was an open-label having patients with type I spinal muscular atrophy. Part 1 of the study with (n=21) patients deals with one dosage cohort. Patients with a high dosage cohort (n=17) are recommended 0.2 mg/kg/day before 12 months of treatment. All the patients suffer from loss of function of the SMN1 and SMN2 genes. In the part one study, the duration was 14.8 months, and only 19 patients were treated with a minimum duration of 12 months. Among these treated patients, 41 % were recommended 0.2 mg/kg/day and could sit independently without support for at least more than 5 seconds after 12 months of treatment. 25 % were expected to survive without any ventilation patients who were above 14 months of age. And after the treatment of 12 months, 90 % of the patients can survive without ventilation who were 15 months old patients. And after 23 months of treatment with Risdiplam, patients can survive without ventilation in 28 months old patients.
-
Later-Onset Spinal Muscular Atrophy: It was a multicenter trial on patients with SMA Type 2 or 3. Part one is a dose-finding on 51 patients, and part 2 is a randomized, double-blinded, and placebo-controlled study.
Primary Endpoint: The changing phase from baseline to 12 months in the Motor Function Measure 32 score and Revised Upper Limb in Module (RULM) score.
Part two study had 180 patients with Type -2 SMA (71 %) and type 3 (29 %). Patients were randomly receiving Risdiplam as recommended dosage according to age groups (2 to 5, 6 to 11, 12 to 17, 18 to 25 years). The median age is nine years. And the duration of treatment is 102.6 months. 67 % of patients have scoliosis. And the score on MFM32 is 46.1%, and RULM is 20.1%. The primary endpoint changes in total MFM32 score at 12 months with Risdiplam (N=1.36) and placebo (n= -0.19). Secondary endpoints with changes from baseline MFM32 total score of three at 12 months with 38.3 % Risdiplam and 23.7% placebo.
Patient Counseling Information:
-
Advise the patient to go through the FDA (Food and Drug Administration) guidelines.
-
Pregnancy and Fetal Risks: The patient should be informed about the risks and fetal harm the drug can do to pregnant women. So, it is advised to use contraceptives along with Risdiplam.
-
Potential Effects on Male Fertility: Males can also get compromised while getting treated with Risdisplam.
-
Preparation of Oral Solution: The patient should know they will get a liquid form from the pharmacy and not mix the drug with formula milk or any milk while giving it to infants. Use the drug immediately after drawing up.












