Overview
Certolizumab, widely known by its generic name Certolizumab pegol (CZP) is a potent tumor necrosis factor-alpha (TNF-alpha) inhibitor recently used for treating rheumatoid arthritis (RA). The U.S. FDA (the United States Food and Drug Administration) extended the use of Certolizumab pegol to treat moderate to severe forms of rheumatoid arthritis in adult patients on 13th May 2009. Initially, Certolizumab was approved by FDA on 22nd April 2008 to treat Crohn’s disease. Certolizumab is a TNF-alpha neutralizing agent which is pegylated (polyethylene glycol attached) to increase its therapeutic efficacy.
Certolizumab pegol blocks the production of inflammatory mediators and is effective in disrupting inflammatory changes in autoimmune diseases like rheumatoid arthritis (RA). Structurally, Certolizumab is a monoclonal antibody (mAb) that targets TNF-alpha and constitutes only the Fab fragment of the antibody. Its Fc portion is removed to reduce toxic effects in Certolizumab pegol therapy. Polyethylene glycol (PEG) is conjugated to the free cysteine residue of its heavy chain. Pegylation increases the half-life of Certolizumab.
Indications
Certolizumab is an effective tumor necrosis factor (TNF-alpha) blocker that is indicated for treating various autoimmune diseases. Some of the indications for using Certolizumab pegol are as follows:
-
Certolizumab is indicated in Crohn’s disease for reducing its inflammatory signs and symptoms and also maintains optimal clinical response in individuals with active disease who do not respond to conventional treatment.
-
For the treatment of adult patients with active rheumatoid arthritis (RA) who exhibit moderate to severe clinical symptoms.
-
Its use is indicated in adult individuals with active forms of psoriatic arthritis (PsA).
-
For treating active ankylosing spondylitis (AS).
-
For treating active non-radiographic axial spondyloarthritis.
Contraindications
There are no specific contraindications for the use of Certolizumab pegol. However, its use is avoided in some of the following conditions -
-
Not indicated in pediatric patients because of the high risk of developing malignancies like lymphoma.
-
In case of developing hypersensitivity reactions.
-
During immunizations with live and live attenuated vaccines.
-
In case of severe existing infections.
Boxed Warning
Clinical use of Certolizumab pegol contains the highest safety-associated warnings due to the major risks of developing serious infections or malignancies. Some of the precautions included in the boxed warning for Certolizumab pegol are as follows -
-
Increased risk for developing severe infections in individuals who are on concomitant immunosuppressive therapies taking corticosteroids or Methotrexate.
-
Risk of developing active tuberculosis and also reactivation of latent tuberculosis infections.
-
Prone to invasive fungal infections such as histoplasmosis, and pneumocystis.
-
Acquiring bacterial, viral, or other opportunistic infections, particularly in immunocompromised individuals.
-
Chances of developing lymphoma or other fatal malignancies.
Dosage Forms and Available Strengths
Certolizumab pegol is available in two different injectable forms.
-
Lyophilized Powder - To be reconstituted before administration. Certolizumab pegol is manufactured as sterile, white, lyophilized powder packed in single-use vials containing nearly 200 mg of Certolizumab pegol for reconstitution.
-
Prefilled Syringe - 1 mL prefilled glass syringe for single-use containing a fixed half-inch thin-walled 25 gauge needle that provides 200 mg/mL of Certolizumab pegol.
Dosage and Route of Administration
-
Route of Administration - Certolizumab pegol is given as a subcutaneous injection.
-
The Recommended Dose for Rheumatoid Arthritis - 400 mg of Certolizumab pegol for adult patients.
-
Initial doses are given as two 200 mg subcutaneous injections in 2nd week and 4th week followed by 200 mg every other week.
-
Maintenance doses include 400 mg of Certolizumab pegol every four weeks.
Administration of Certolizumab Pegol
Reconstituting Lyophilized Powder
-
Reconstitution of lyophilized powder is done and injected by only a trained healthcare professional.
-
The package contains all the required components to be reconstituted and injected.
-
The vials containing Certolizumab pegol lyophilized powder are removed from the refrigerator and kept for 30 minutes to attain room temperature before reconstituting.
-
The vial should not be warmed by any other method and sterile techniques to be followed during the preparation and administration of Certolizumab pegol.
-
1 ml of sterile water is introduced into the vial along the wall (direct adding is avoided) with the appropriate needle provided.
-
The vial is gently swirled for nearly one minute without shaking to ensure complete contact between the powder and the sterile water. The foaming effect should be avoided.
-
Swirling is continued every five minutes till no undissolved particles are present. The entire reconstitution procedure takes about 30 minutes.
-
200 mg/mL of Certolizumab pegol reconstituted solution is finally obtained which is colorless to pale yellow, clear to opalescent liquid free of particles.
-
After reconstitution, the final solution could be stored for up to 24 hours in the vials at a temperature between two to eight degrees Celsius, but should not be frozen.
Administering Through Subcutaneous Injection
-
Before injecting, the reconstituted Certolizumab pegol is allowed to reach room temperature, but should not remain more than two hours at room temperature prior to injection.
-
A new 20-gauge needle with a separate syringe is used to withdraw the reconstituted solution, thereby a single syringe contains 1 mL of Certolizumab pegol (200 mg/mL).
-
For subcutaneous administration, the 20-gauge needle on the syringe is replaced with a 23-gauge needle.
-
The skin of the abdomen or thigh is pinched and the full contents in the syringe are injected subcutaneously.
-
If the recommended dose is 400 mg of Certolizumab pegol, two injections of 200 mg each are given at two separate sites.
Using Certolizumab pegol Prefilled Syringe
-
For self-injecting prefilled Certolizumab pegol syringe, proper training is given in the subcutaneous injection technique.
-
Patients are allowed to use prefilled syringes only upon the physician’s approval.
-
Patients are instructed to inject the entire content (1 mL) from the prefilled syringe.
-
The directions for use and instructions for using Certolizumab pegol prefilled syringes are provided in the standardized booklet.
Precautionary Measures
-
Certolizumab pegol therapy should be regularly monitored for the development of serious infections. In case of infection or sepsis, its use is discontinued and reassessed.
-
Lymphoma and other malignancies could develop in patients receiving Certolizumab pegol.
-
Reactivation of the hepatitis B virus is highly suspected during Certolizumab pegol therapy. If reactivation is seen, Certolizumab pegol is stopped, and antiviral therapy is initiated.
-
Cytopenias or pancytopenia occurs during Certolizumab pegol treatment.
For Patients
What Is Rheumatoid Arthritis?
Rheumatoid arthritis (RA) is a chronic, inflammatory disease with systemic manifestations. Though its cause is not known, complex genetic, as well as environmental factors contribute to its pathogenesis resulting in inflammation of the synovial tissues and bone damage. Primarily joints are affected leading to fatigue, pain, and reduction in joint movement.
The predominant clinical symptoms are swollen painful joints and stiffness. Other extra-articular sites that are affected include the lungs and heart. Anemia, neuropathy, ocular diseases, and vasculitis also occur. Untreated or uncontrolled inflammation causes irreversible joint damage, leading to functional disability and reduced quality of life.
How Is Certolizumab Pegol Effective in Rheumatoid Arthritis?
Biological agents such as monoclonal antibodies have proven effective in the management of rheumatoid arthritis (RA). TNF-alpha is the primary pro-inflammatory mediator that increases inflammation in rheumatoid arthritis. Certolizumab pegol is an effective tumor necrosis factor-alpha (TNF-alpha) block that reduces inflammation in rheumatoid arthritis. It is a monoclonal antibody that blocks the activity of TNF-alpha and reduces joint inflammation.
What Are the Adverse Reactions Caused by Certolizumab pegol?
The adverse reactions observed during Certolizumab pegol are widely associated with its effect on the immune system resulting in an increased chance of developing infections. Some of its adverse reactions are listed below -
-
Serious infections.
-
Malignancies.
-
Heart Failure.
-
Upper respiratory infections.
-
Rash.
-
Urinary tract infections.
-
Anemia.
-
Leukopenia (reduced number of white blood cells).
-
Lymphadenopathy (enlargement of lymph nodes).
-
Pancytopenia (decrease in the number of red blood cells, white blood cells, and platelets).
-
Thrombophilia (increase in the number of platelets).
-
Headache.
-
Hypertension.
-
Nasopharyngitis (presence of swelling in the nasal passage and at the back of the throat).
-
Back pain.
-
Pyrexia (raised body temperature).
-
Pharyngitis (inflammation of the upper throat region).
-
Acute bronchitis (inflammation of the bronchial tubes).
-
Fatigue.
-
Angina pectoris (a type of chest pain caused by reduced blood flow to the heart).
-
Arrhythmias (irregular heartbeat).
-
Atrial fibrillation (abnormally rapid heart rhythm).
-
Pericardial effusion (collection of fluids in tissues surrounding the heart).
-
Dermatitis (severe skin irritation).
-
Urticaria (itchy rashes on the skin).
What Are the Precautions to Be Taken During Certolizumab Pegol Therapy?
The precautions to be followed during Certolizumab pegol are as follows -
-
Both forms of injections (after reconstitution of lyophilized powder and prefilled syringe) should be a clear solution and free of turbidity.
-
The injection should be discarded if there is the presence of impurities or chalky sediments.
-
Recent history of immunizations should be reported before starting Certolizumab pegol therapy.
-
Any allergic reaction or hypersensitivity reaction developing after injection should be reported immediately.
For Doctors
Pharmacodynamics
Certolizumab pegol (CZP) is a humanized anti-TNF-alpha monoclonal antibody that contains only the antigen-binding fragment (Fab). This fragment is made of both light chains and heavy chains with a molecular weight of nearly 90.8 kDa. The Fab fragment is target specific for human TNF-alpha and its dissociation constant (KD) is approximately 90 pM.
TNF-alpha is a major proinflammatory cytokine that induces inflammatory changes in rheumatoid arthritis (RA). It is predominantly present in synovial fluids and synovial membranes resulting in damage to the joints. Certolizumab pegol neutralizes the inflammatory activity of TNF-alpha.
Preparation of Certolizumab Pegol
-
The monoclonal antibodies of Certolizumab pegol are prepared by recombinant sequencing in Escherichia coli (nonhuman protein sequence) where the final product is target specific for human TNF-alpha.
-
The humanized monoclonal antibodies of Certolizumab pegol contain only Fab fragments which are essential for binding with antigens present in TNF-alpha.
-
The Fc portion in an antibody is responsible for complement-dependent reactions.
-
This portion is removed in Certolizumab pegol to eliminate complement-mediated cytotoxicity (CDC) as well as antibody-dependent cell-mediated cytotoxicity (ADCC), thereby minimizing side effects due to monoclonal antibody therapy.
Mechanism of Action
-
Certolizumab pegol is highly specific for human TNF-alpha and binds with increased affinity with both soluble and membrane-bound forms of TNF-alpha.
-
The antigen binding of Certolizumab pegol selectively neutralizes TNF-alpha.
-
TNF-alpha plays a key role in inflammatory processes by activating the production of inflammatory mediators like prostaglandins, interleukin-1, nitric oxide, and platelet-activating factor.
-
Increased TNF-alpha levels in the synovial fluid during rheumatoid arthritis contribute to joint destruction.
-
Certolizumab pegol increasingly penetrates the inflamed tissue in rheumatoid arthritis to a greater extent and Inhibits the inflammatory activity of TNF-alpha.
-
Neutralizing TNF-alpha and disrupting the production of inflammatory mediators contributes to the effectiveness of Certolizumab pegol in treating rheumatoid arthritis.
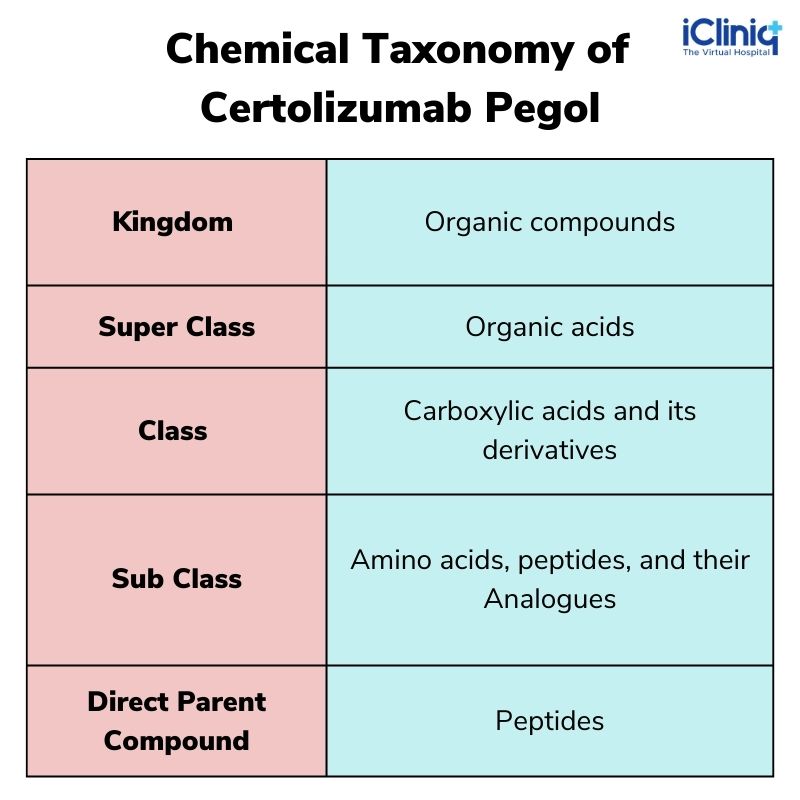
Chemical Taxonomy of Certolizumab Pegol
Pharmacokinetics
-
After subcutaneously administering Certolizumab pegol, its peak plasma concentrations are obtained between 54 to 171 hours.
-
Its bioavailability ranges from 76 % to 88 %.
-
The Pegylation of Certolizumab contributes to its increase in half-life which is up to 14 days.
-
Distribution - Its steady-state volume of distribution is 6 to 8 L.
-
The maximum plasma concentration (Cmax) and the area under the concentration versus time Curve (AUC) of Certolizumab pegol are dose-dependent.
-
Due to the absence of the Fc fragment, it does not cross the placenta.
-
The site where the metabolism of Certolizumab pegol occurs is not yet identified.
-
Elimination - Renal excretion is the primary route of elimination.
-
The clearance rate of Certolizumab pegol is estimated to be 21.0 mL/h and complete elimination takes up to five months.
-
Clinical Efficacy - The efficacy and safety of Certolizumab pegol are carefully assessed through clinical trials and these reports suggest its use in monotherapy as well as in combination with disease-modifying anti-rheumatic drugs (DMARDs) for treating active forms of rheumatoid arthritis.
Toxicities
Certolizumab pegol is well tolerated and does not cause immunogenic side effects due to the removal of Fc fragments. The commonly reported toxic effects are due to the development of infection or malignancies. Some of the toxic side effects of Certolizumab pegol therapy are as follows -
-
Pneumonia.
-
Cellulitis.
-
Urinary tract infection.
-
Hepatitis B virus reactivation.
-
Pyrexia, urticaria, and rash.
-
Hypersensitivity reactions such as angioedema, and injection site reactions.
-
Neurologic reactions like neuritis, and demyelinating disorders.
-
Hematologic reactions such as anemia, leukopenia, and clotting disorders.
-
Development of autoimmune disorders such as lupus-like syndrome.
-
Skin disorders such as dermatitis, and erythema nodosum.
-
Development of lymphomas such as B-cell lymphoma, Hodgkin's disease,non-Hodgkin's lymphoma, and mycosis fungoides.
Drug Interactions
-
Live vaccines and live attenuated vaccines are not given during Certolizumab pegol therapy.
-
The use of Certolizumab pegol in combination with other immunomodulators such as Anakinra, Rituximab, Abatacept, or Natalizumab is not recommended due to the increased risk of infections.
-
Certolizumab pegol interferes with coagulation tests resulting in elevated activated partial thromboplastin time (aPTT) without any clinical coagulation abnormality.
-
Concomitant use of Certolizumab pegol with oral corticosteroids leads to gastrointestinal perforations.
-
Certolizumab pegol does not cause significant reactions in concomitant use with Methotrexate and disease-modifying anti-rheumatic drugs (DMARDs).
Conclusion
Certolizumab pegol is a novel TNF-alpha inhibitor that is effective in reducing inflammation in active rheumatoid arthritis. The drug is well tolerated and shows significant clinical improvement. It is useful as monotherapy as well as in combination therapies.












