Drug Overview
Faricimab-svoa is a drug used in the treatment of age-related macular degeneration (AMD). It is available in solution form for injections. Faricimab-svoa injections are also recommended for patients with diabetes-induced macular edema (DME). Macular edema can result in blurring of vision or loss of vision. It is available in the form of a solution, as it is taken only under a doctor's supervision. The drug Faricimab was approved by FDA (Food and Drug Administration) in 2022 for the management of neovascular age-related macular degeneration and diabetic macular edema. Read the article to know in detail about Faricimb-svoa uses, indications, contraindications, side effects, and mechanism of action.
Uses of Faricimab-Svoa
Faricimab-svoa is an approved drug for the management of :
-
Neovascular age-related macular degeneration (nAMD)
-
Diabetes-induced macular edema (DME).
Doses
-
It is available in the form of a solution for injections in the dose of 6 milligrams per 0.05 milliliters strength.
Warnings
-
Risk of Vision Problems: There is an increased risk of loss of vision or blurring of vision with regular usage of the medicine. The drug Faricimab carries risk of retinal detachments and endophthalmitis after the administration of the drug. It is advisable to report to the doctor if a sudden blurring of vision occurs after starting the drug.
-
Risk of Cardiovascular Disease: The drug Faricimab carries a risk of blood clot formation, Heart stroke, and heart attack. It is suggested to report to the doctor urgently if there are any signs of pain in the legs, chest, and groin.
-
Risk of Increase in Intraocular Pressure: There is an increased risk of raised intraocular pressure on the use of the drug Faricimab within 60 minutes. Continuous monitoring of intraocular pressure is advised simultaneously after the administration of the drug.
-
Risk of Immunogenicity: The drug Faricimab has the potential to cause immunogenicity. Patients should inform the doctor soon in case of eye pain, light sensitivity in the eyes, and vision loss.
-
Bilateral Treatment: The safety of the use of Flaricimab in both eyes simultaneously has not been yet established.
For Patients:
What Is Age-Related Macular Degeneration?
Age-related macular degeneration (AMD) is a disease characterized by blurring or loss of vision due to the degeneration of the retina. It mostly affects people above the age of 50. Macular degeneration can be dry or wet. In the case of dry macular degeneration, the center of the retina deteriorates, while in wet macular degeneration, the blood vessels grow under the retina. Wet macular degeneration is a more severe form than the dry form. Wet macular degeneration has two further subtypes:
-
Occult macular degeneration.
-
Classic macular degeneration.
Various risk factors associated with age-related macular degeneration are:
-
Smoking.
-
Underlying systemic diseases like diabetes.
-
Obesity.
-
High blood pressure.
-
Side effects of the other drug.
-
The lighter color of eyes.
Learn More About Faricimab-Svoa:
When and Why to Take Faricimab-Svoa?
-
Faricimab-svoa is prescribed by the ophthalmologist (eye specialist) to patients for the management of age-related macular degeneration (wet) and macular degeneration induced by diabetes.
-
The drug Faricimab is available in solution forms that are given as a shot into the eye every four weeks (about every 28 days). A minimum of 4 doses are given.
-
Any additional extension of the doses is decided by the doctor based on the patient's condition.
Things to Tell the Doctor Before They Prescribe Faricimab-Svoa:
-
Inform the doctor in case of any allergy to any drug or its ingredients.
-
Inform the doctor about any surgical history if present.
-
In case of any systemic diseases like diabetes, hypertension, bleeding disorders, or skin disease it is advised to inform the doctor prior to starting medications.
-
Inform the doctor in case of any eye infections.
-
Tell the doctor in case of pregnancy or planning pregnancy.
Starting Faricimab-Svoa:
-
Make sure to visit the doctor or healthcare professional to get the Faricimab injections shots into the eyes regularly at the specific schedule as suggested by the doctor so as to avoid missing the dose.
-
Faricimab injections are usually administered by the eye specialist (ophthalmologist) every twenty-eight days for at least four doses.
-
In case if unable to visit the doctor, inform the doctor prior.
-
Keep follow-up with the doctor regarding the drug schedules.
Things to Do After Start Taking Faricimab-Svoa:
-
Inform the doctor in case of any itching or irritation in the eyes for a longer duration after the medication.
-
Make sure to inform the doctor that blurring of vision or cloudy vision occurs after starting the drug.
-
Make a note of improvement in the symptoms and inform the doctor during the follow-up visits.
Look Out for the Side Effects:
There are various unwanted side effects associated with the drug Faricimab-svoa:
Common side effects:
-
Redness in the eyes.
-
The blurring of the vision.
-
Blood in the eyes.
-
Tenderness in the eyes.
-
Sore eyes.
-
Loss of vision.
-
Presence of dark spots in front of the eyes.
Serious side effects:
-
Light flashes.
-
Watering of eyes.
-
Fever.
-
Hives.
Dietary Alterations: There is no specific diet alteration needed when on Faricimab medication.
What Should Be Done If a Dose Is Missed?
If a dose is missed, inform the doctor immediately and get the next appointment scheduled as soon as possible.
Avoid Self-Prescription:
Faricimab-svoa is prescribed and given by an eye specialist only. Avoid self-prescription of the drug.
For Doctors:
Indications
-
Neovascular age-related macular degeneration (nAMD).
-
Diabetes-induced macular edema (DME).
Contraindications
-
Ocular or Periocular Infections: The drug Faricimab should be strictly avoided in patients with ocular or periocular (within or around the eyes) infections.
-
Active Intraocular Swelling: The drug should not be given in patients with active swelling in or around the eyes as it can make the condition worse.
-
Hypersensitivity: Patients allergic to the drug or its composition should not be administered the drug.
Adverse Reactions Following Administration of the Drug Faricimab
Various adverse reactions following the administration of the drug Faricimab are:
-
Conjunctival Hemorrhage: It is the most common adverse event observed following the administration of the drug Faricimab.
-
Vitreous floaters: Black or gray specs like spots in the vision.
-
Increase Intraocular Pressure: Increases in the pressure within the eyes have been observed in many patients following the administration of the drug Faricimab within 60 minutes of administering the drug.
-
Eye Irritation: Watery eyes with scratching or itching in the eyes are observed.
-
Retinal Pigment Tear: Tear in retinal pigment epithelium.
-
Vitreous Hemorrhage: Accumulation of blood in the vitreous cavity.
Pharmacology:
Mechanism of Action: Faricimab is a bispecific antibody (bsAb) consisting of two light chains and two heavy chains. It acts by suppressing endothelial growth, neovascularization, and vascular permeability, which are responsible for increasing the retinal thickness, which is observed in age-related macular degeneration and diabetes-induced macular edema.
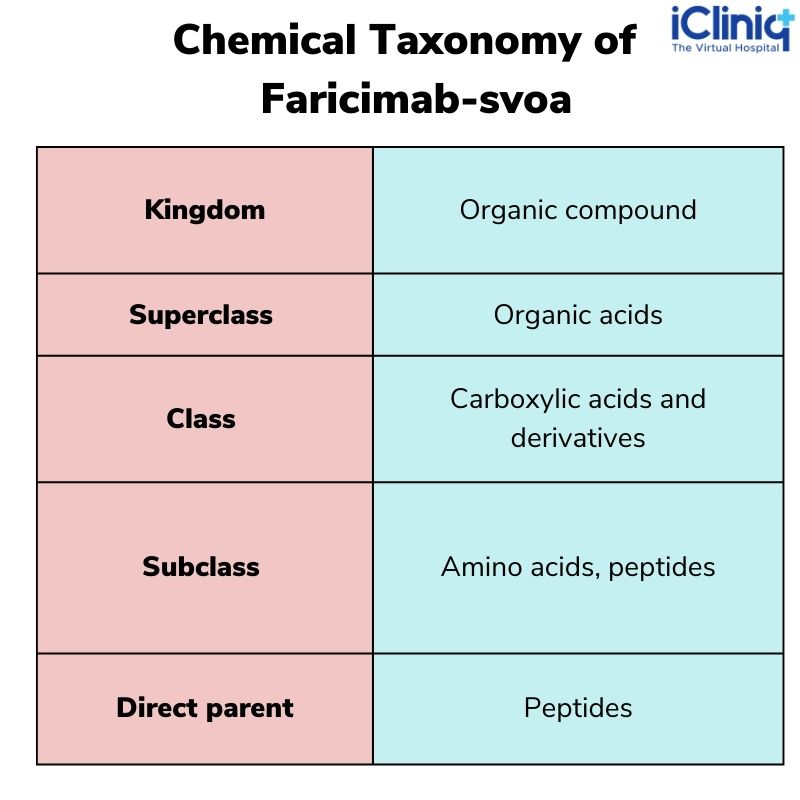
Chemical Taxonomy:
Ingredients:
Active Ingredient: Faricimab is the only active ingredient present in the drug.
Inactive Ingredient:
-
Histidine.
-
Methionine.
-
Sodium chloride.
-
Sucrose.
-
Polysorbate.
-
Water.
-
Acetic acid.
Absorption: The drug Faricimab has an average plasma concentration of 0.23 micrograms per milliliter. The peak plasma time is around 2 days.
Metabolism: Faricimab is catabolized like endogenous immunoglobulins.
Elimination: Faricimab is eliminated through the kidneys after its breakdown into amino acids and smaller peptides.
Half-Life: The average estimated half-life of Faricimab is around 7.5 days.
Shelf-Life: The average shelf-life of the drug is around 30 months.
Doses and Forms:
1. Faricimab is available in the solution form and is given by healthcare professionals in injection form. One of the following regimens can be followed:
-
6 mg of the drug by intravitreal injection at weeks 28 and 44.
-
6 mg of the drug by intravitreal injection at weeks 24, 36, and 48.
-
6 mg of the drug by intravitreal injection at weeks 20, 28, 36, and 44.
2. The drug Faricimab is a colorless or brownish-yellowish solution.
3. It has a pH (potential of hydrogen) of 5.5.
4. The osmolality of a drug is around 270 to 370 mOsm per kilogram.
Overdose: In case an extra drug is administered or deposited in the eyes or overdoses may result in an increase in intraocular pressure. Continuous monitoring of intraocular pressure is recommended in case of overdose.
Drug Interactions: There are possible effects of combining two drugs on their concentration, absorption, metabolism, and excretion. There is currently no information available on the drug interaction of Faricimab.
Storage:
-
The drug Faricimab should be kept at 2 to 8 degrees Celsius. If removed from the refrigerator it can also be stored at a normal room temperature of 20 to 25 degrees Celsius.
-
It is advised not to freeze or shake the drug.
-
Protect the drug from the light.
-
Avoid using the drug if the solution is discolored or shows cloudiness.
-
Avoid using the drug if it is expired.
-
Administered the drug using aseptic precautions.
Administration of the Drug Faricimab-Svoa:
-
Intravitreal Faricimab injections should be administered to patients by healthcare professionals only.
-
Standard aseptic precautions should be taken while administering the injection to prevent infections.
-
An adequate amount of anesthesia must be administered before administering the drug.
-
After the administration of the intravitreal Faricimab, the intraocular pressure of the eyes should be monitored simultaneously.
-
The drug should be administered very slowly.
-
A single syringe should be used for one eye. A new syringe should always be used if the contralateral eye also needs to be treated.
-
Monitor the patient's eyes for any signs of redness, retinal detachment, eye pain, and vision loss.
Faricimab for Neovascular Age-Related Macular Degeneration (nAMD)
-
Initiation Dose: The drug Faricimab is administered intravitreally in the affected eye once every twenty-eight days for the first four doses.
-
Maintenance Doses: After eight to twelve weeks of the initial dose the maintenance therapy is started following three regimes:
-
For weeks 28 and 44 every 16 weeks.
-
Weeks 24, 36, and 48 for every 12 weeks.
-
Weeks 20, 28, 36, and 44 for every 8 weeks.
Faricimab for Diabetic Macular Edema
-
The initial dose of 6 mg intravitreally should be administered to the patient once every twenty-eight days for a minimum of four doses. Macular edema is then checked for resolved swelling by optical coherence tomography.
Other Specifications:
-
Faricimab-Svoa in Pregnant and Lactating Women: There is no specific research data available on the safety of the use of the drug Faricimab in pregnant and lactating mothers. The drug Faricimab should be avoided in pregnant and breastfeeding women. According to the clinical trials done in an animal study the drug Faricimab if administered in the period of organogenesis has shown a raised incidence of abortions and birth defects.
-
Faricimab-Svoa in Geriatric Patients: The drug should be used cautiously in patients with systemic diseases like bleeding disorders or cardiac disorders.
-
Faricimab-Svoa in Pediatric Patients: The safety and efficacy of the use of the drug Faricimab have not yet been determined in younger children. There is a lot of research needed to be done to understand the effectiveness of Faricimab in younger people.
-
Faricimab-Svoa in Patients with Renal Impairment: The dose of the drug Faricimab should be mild to moderately adjusted or modified based on the patient's condition.












