Introduction
Gilteritinib is an anti-cancer drug particularly used to manage specific forms of acute myeloid leukemia. The drug works as an FLT3 or tyrosine kinase inhibitor. The drug is beneficial to patients in whom cancer has relapsed or returned and are unresponsive to other treatment options. Leukemia is a cancer that primarily affects the body's white blood cells. The drug is recommended for acute myeloid leukemia (AML) patients associated with FLT3 gene mutation. Mutations or alterations in the genetic structure affect the working of genes. People with AML must get tested for FLT3 gene mutations. As Gilteritinib belongs to the class of tyrosine kinase inhibitors, it works by targeting and attacking cancer cells. Gilteritinib was developed by Astellas Pharma and granted the orphan drug designation by the United States (US) Food and Drug Administration (FDA) on July 20, 2017. Finally, Gilteritinib was approved by the FDA on November 28, 2018.
Safety and Effectiveness of Gilteritinib:
A clinical study was performed to compare the efficacy of Gilteritinib to other chemotherapeutic agents. In addition, the study aimed to look for complete cancer remission. Hence, a trial named ADMIRAL was done on 138 adults with relapsed or refractory AML due to FLT3 gene mutation. The patients were given Gilteritinib 120 mg orally until acceptable toxicity. After a follow-up of 4.6 months, 29 patients had complete remission along with partial hematologic recovery. The other 106 patients became independent of RBC (red blood cells) or platelet transfusion during a period of 56 days. The common adverse reactions reported by trial participants were:
-
Malaise.
-
Fever.
-
Fatigue.
-
Nausea.
-
Stomatitis.
-
Edema.
-
Rashes.
-
Cough.
-
Headache.
-
Vomiting.
-
Dizziness.
How Does Gilteritinib Work to Treat AML?
AML is a type of cancer that affects the white blood cells in the bone marrow. It commonly occurs due to mutations in the FLT3 gene or tyrosine kinase. This is the time when Gilteritinib comes into action. As the drug is a small molecule tyrosine kinase inhibitor, it inhibits the tyrosine kinase receptors, including FLT3. Certain cells express FLT3, including tyrosine kinase domain mutations (FLT3-D835) and FLT3-ITD-D835Y. Gliterititnib particularly inhibits FLT3 receptors and the signaling pathways that stimulate their proliferation. In addition, the drug also induces cell death in leukemic cells, which express FL3-ITD.
How Long Does Gilteritinib Take to Work?
The onset of duration varies from one individual to the other. For example, people who are responsive might respond within two months. However, the drug's action and efficacy depend on the body and medical history. The following factors affect the duration of the onset of drug action:
-
The severity of the patient's condition.
-
The previous treatment the patient has undergone for a particular condition.
-
The type of FLT3 mutations one experiences.
The doctor can check the drug's effectiveness by measuring the number of cancer cells in the body. The patient might show significant improvements within a few months. However, anti-cancer drugs have several associated side effects. Studies report that some patients developed a response within 27 days after taking Gilteritinib. It means that cancer symptoms have lessened over time, along with the partial recovery of blood count.
For Patients:
Acute Myeloid Leukemia (AML):
AML is a cancer that initiates in the blood-forming cells of the bone marrow. It has been named acute because of its rapid speed of progression. As cancer is characterized by the rapid proliferation of body cells, the blood cells divide and move out of the bloodstream quickly in AML. Sometimes, cancer can spread to other body parts, including the liver, lymph nodes, spleen, brain, spinal cord, or testicles. Several factors, including smoking, radiation therapy, and chemotherapy, increase the risk of AML.
Signs and Symptoms of AML:
The general signs and symptoms of AML are listed below:
-
Bone pain.
-
Fever.
-
Fatigue.
-
Shortness of breath.
-
Pale skin.
-
Increase in the frequency of infections.
-
Bleeding from the nose or gums.
What Is the Most Important Information One Should Know About Gilteritinib?
Gilteritinib must be used cautiously as it might cause some serious side effects listed below:
-
Differentiation Syndrome - It is a life-threatening condition that affects the blood cells. Differentiation syndrome is usually noticeable within two days of starting the Gilteritinib therapy and during the first three months of treatment. Hence, the patient must consult the doctor or visit the emergency room immediately if he notices the following symptoms of differentiation syndrome:
-
Fever.
-
Cough.
-
Respiratory difficulties.
-
Rashes.
-
Lightheadedness.
-
Dizziness.
-
Weight gain.
-
Swelling of the arms or legs.
-
Decreased urine frequency.
-
What Should the Patient Inform the Doctor Before Taking Gilteritinib?
Before taking Gilteritinib, the patient must inform the doctor if he or she has the following:
-
Heart disease, including long QTc syndrome.
-
Abnormal levels of electrolytes, including sodium, potassium, calcium, or magnesium.
-
Conceived or planned for the same in the future. Gilteritinib can harm the unborn baby, so one must avoid getting pregnant during the therapy.
-
For patients planning to become pregnant, the doctor might perform a pregnancy test seven days before the treatment.
-
Females must use appropriate birth control measures during Gilteritinib therapy for six months after the last dose.
-
Males with female partners that can become pregnant must use birth control measures for four months after the last Gilteritinib dose.
-
-
Breastfed or planning for the same. Not much information is available regarding the presence of Gilteritinib in breast milk. Hence, one must avoid breastfeeding for two months after the last Gilteritinib dose.
How Should the Patient Take Gilteritinib?
-
Take Gilteritinib exactly as directed by the doctor.
-
Avoid changing the drug dose or stop taking it without consulting the doctor.
-
Gilteritinib tablets must be swallowed as a whole. Avoid breaking, crushing, or chewing them.
-
The drug can be consumed with or without food.
-
For people who missed the drug or did not take it at the scheduled time, they must take the drug soon and 12 hours before the next dose.
-
Follow the normal schedule for the next dose. However, avoid taking two doses within 12 hours.
What Are Some of the Side Effects of Gilteritinib?
Gilteritinib can cause some of the following serious side effects:
-
Posterior Reversible Encephalopathy Syndrome (PRES) - People taking Gilteritinib are at a higher risk of developing PRES, which affects brain cells. Hence, the patient must inform the doctor immediately in case of the following symptoms:
-
Headache.
-
Decreased alertness.
-
Confusion.
-
Eyesight problems.
-
Blurred vision.
-
The doctor will perform a test to evaluate the symptoms of PRES and might recommend the patient discontinue the drug.
-
QTc Prolongation - Gilteritinib can induce changes in the electrical activities of the heart resulting in QTc prolongation. Hence, the doctor will evaluate the patient's ECG (electrocardiogram) for the electrical activity of the heart before and during Gilteritinib therapy. The patient must inform the doctor in case of dizziness, lightheadedness, or fainting. There is a higher risk of QT prolongation in people with depressed blood potassium and magnesium levels.
-
Pancreatitis - Patients with pancreatitis or inflammation of the pancreas have stomach pain that does not subside easily. The pain might be accompanied by vomiting or nausea.
Some of the other common side effects of Gilteritinib are listed below:
-
Muscle or joint pain.
-
Changes in liver function tests.
-
Fever.
-
Tiredness.
-
Pain or sores in the mouth or throat.
-
Diarrhea.
-
Nausea.
-
Cough.
-
Shortness of breath.
-
Constipation.
-
Headache.
-
Dizziness.
-
Low blood pressure.
-
Eye problems.
-
Decreased urination.
For Doctors:
Indications and Usage:
Gilteritinib has been approved by the FDA for managing relapsed or refractory acute myeloid leukemia with mutations in the FLT3 gene.
Contraindications:
Gilteritinib must be avoided in patients with allergies to its ingredients.
Dosage and Administration:
Patient Selection:
The ideal candidates for Gilteritinib therapy are those with AML and mutations in the FLT3 gene in the bone marrow or blood. The response might be delayed. The drug must be given for six months in the absence of disease progression or unacceptable toxicity to allow time for response.
Recommended Dosage:
The recommended initial dose of Gilteritinib is 120 mg once daily, with or without food. The response might be delayed. Avoid crushing or breaking the tablets, and take them at the same time daily. In case of a missed dose, the drug must be taken on the same day and 12 hours before the next dose.
Dosage Modifications:
The patient's blood count and creatinine phosphokinase levels must be assessed before the initiation of the therapy. The count must be checked once weekly or monthly, depending on the severity. The dosage modifications based on adverse reactions are mentioned as below:
1. Differentiation syndrome: The patient must be given systemic corticosteroids with hemodynamic monitoring until the symptoms subside for at least three days. Interrupt the therapy if symptoms persist for 48 hours or above. Resume the therapy only when the symptoms improve to grade 2.
2. Posterior reversible encephalopathy syndrome: Discontinue the drug.
3. QTc interval above 500 milliseconds: Interrupt Gilteritinib therapy. Resume the therapy only when the QTc interval is between 30 to 480 milliseconds.
4. QTc interval above 30 msec on ECG on day eight of cycle 1 - Perform an ECG on day nine. Reduce the dose to 80 mg.
5. Grade 3 or higher toxicity - Interrupt the therapy until toxicity subsides and then resume at 80 mg.
6. Pancreatitis - Interrupt the therapy until pancreatitis subsides and then resume at 80 mg.
Contraindications:
Gilteritinib is specifically contraindicated in patients with hypersensitivity to any of its ingredients. Some patients have experienced anaphylactic reactions during clinical trials.
Warnings and Precautions:
-
Differentiation Syndrome - Differentiation syndrome was reported in 3 % of patients out of the 319 trial participants. This syndrome is characterized by rapid differentiation and proliferation of myeloid cells and can be fatal or life-threatening. The common symptoms of differentiation syndrome in people treated with Gilteritinib are listed below:
-
Fever.
-
Dyspnea.
-
Pericardial effusion.
-
Pleural effusion.
-
Hypertension.
-
Weight gain.
-
Pulmonary or peripheral edema.
-
Rashes.
-
Renal dysfunction.
-
Concomitant acute febrile neutrophilic dermatosis.
-
Differentiation syndrome was reported as early as two days or 75 days after the therapy. It has been observed with or without leukocytosis. Patients with differentiation syndrome must be given Dexamethasone 10 mg intravenously every 12 hours. In addition, hemodynamic monitoring is required until improvement. Corticosteroids can be tapered after the resolution of symptoms. Gilteritinib therapy must be stopped if the symptoms persist for more than 48 hours.
-
Posterior Reversible Encephalopathy Syndrome - Out of the 319 patients treated with Gilteritinib, 1 % of them experienced posterior reversible encephalopathy syndrome, including seizures and altered mental status. However, the symptoms got resolved after discontinuing Gilteritinib therapy. Hence, magnetic resonance imaging (MRI) can be done to confirm the diagnosis.
-
Prolonged QT Interval - Gilteritinib can be associated with prolonged cardiac ventricular repolarization or QT interval prolongation. During the clinical trial, 1 % of patients had an increase in the QTc interval. Hence, an ECG must be performed on days 8 and 15 of the first cycle and before the initiation of the next cycle.
-
Pancreatitis - Out of the 319 patients, 4 % of them reported pancreatitis or inflammation of the pancreas. Hence, dosage adjustments must be made in such patients.
-
Embryo-Fetal Toxicity - Based on the mechanism of action, Gilteritinib can cause problems in the embryo or fetus. When the drug was administered in animals during organogenesis, teratogenicity and suppressed fetal growth were observed. Hence, females of reproductive potential must be counseled to use effective birth control methods during the therapy and four months after the last dose.
Description:
Gilteritinib is particularly a tyrosine kinase inhibitor. The chemical name of the drug is 2-Pyrazinecarboxamide, 6-ethyl-3-[[3-methoxy-4 [4-(4-methyl-1-piperazinyl)-1-piperidinyl] phenyl] amino]-5-[(tetrahydro-2H-pyran-4-yl) amino]-, (2E)-2 butenedioate (2:1). In addition, the molecular weight of Gilteritinib is 1221.50, and the molecular formula is (C29H44N8 O3)2. The drug is light yellow and sparingly soluble in water. In contrast, the drug is slightly soluble in anhydrous ethanol.
Clinical Pharmacology:
Mechanism of Action:
Gilteritinib is a small molecule that works against multiple receptor tyrosine kinase or FMS-like tyrosine kinase 3. The drug has the ability to inhibit the FLT3 signaling pathway, the exogenous proliferation of cells, and tyrosine kinase domain mutations (TKD) FLT3-D835Y and FLT3-ITD-D835Y. In addition, the drug induces apoptosis in leukemic cells showing FLT3-ITD.
Pharmacodynamics:
When Gilteritinib 120 mg was administered in patients with refractory or relapsed AML, 90 % of the inhibition of FLT3 phosphorylation occurred within the first 24 hours.
Cardiac Electrophysiology:
The effects of Gilteritinib were evaluated in patients with prolonged QTc intervals. However, no clinically significant differences were observed in the QTc interval. Out of the 317 patients, 1.3 % reported an increase in QT interval.
Pharmacokinetics:
Absorption:
The maximum time of Gilteritinib concentration is observed between four and six hours after administration.
Effect of Food:
When Gilteritinib was administered in a healthy adult with a high-fat meal, the maximum concentration decreased by 26 %, whereas the area under the curve decreased by 10 %.
The median time was delayed by two hours.
Distribution:
The central and peripheral volumes of distribution are 1092 liters and 1100 liters, respectively, which usually indicates that the drug is excessively bound to tissues.
Elimination:
The half-life of Gilteritinib is approximately 113 hours, and the clearance is 14.85 liters per hour.
Metabolism:
The drug is particularly metabolized by CYP3A4. The primary metabolites include M17, M16, and M10, which are formed by N-dealkylation and oxidation.
Excretion:
64.5 % of the total administered dose was recovered in feces, whereas 14.6 % of the radiolabeled dose was recovered in urine.
Composition:
Active Ingredients - Gilteritinib.
Inactive Ingredients - Ferric oxide, hypromellose, mannitol, magnesium stearate, talc, polyethylene glycol, hydroxypropyl cellulose, low-substituted hydroxypropyl cellulose, and titanium dioxide
Chemical Taxonomy of Gilteritinib:
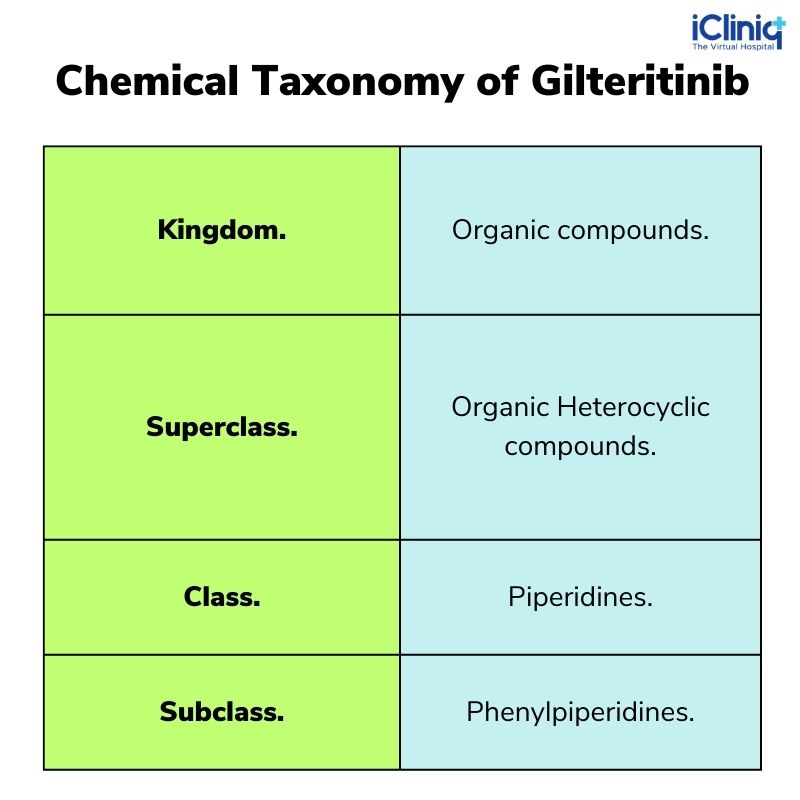
Non-Clinical Toxicology:
Carcinogenesis, Mutagenesis, and Impairment of Fertility:
Ames assay was done to evaluate the mutagenic potential of Gilteritinib. However, the drug did not show any mutagenic potential. Nothing has been known about the effects of Gilteritinib on human fertility.
Adverse Reactions:
-
Myalgia.
-
Arthralgia.
-
Fatigue.
-
Fever.
-
Malaise.
-
Edema.
-
Increased transaminase levels.
-
Constipation.
-
Mucositis.
-
Nausea.
-
Abdominal pain.
-
Febrile neutropenia.
-
Rashes.
-
Dyspnea.
-
Cough.
-
Neuropathy.
-
Dizziness.
-
Headache.
-
Decreased sodium and calcium levels.
Use in a Specific Population:
Pregnancy:
Gilteritinib can cause fetal harm in a pregnant female. However, insufficient data is available regarding the same.
Lactation:
Nothing has been known about the presence of Gilteritinib in human milk. However, traces of the drug were found in milk during animal studies. Hence, females must avoid breastfeeding during Gilteritinib therapy.
Geriatric Population:
No clinically significant changes were observed during the clinical trial in the geriatric population.
Clinical Trial:
A trial named ADMIRAL was done on 138 patients to evaluate the effectiveness of Gilteritinib based on complete remission and partial recovery. The trial participants were kept on follow-up for 4.6 months. The rate of complete remission was 29 patients out of 126 with FLT3-ITD or FLT3-ITD/TKD. Out of the 106 patients, 31 % became free of RBC and platelet transfusions.












