Introduction:
Vaccines have proved to be game-changers in the fight against COVID-19 (coronavirus disease). Adults, babies, and young kids can stay healthy if the vaccine is administered at the right time. There has been considerable progress in the development of vaccines which has made everyone’s lives safe. The World Health Organization (WHO) has approved certain vaccines for COVID-19, which can be safely administered to children six months old and above. The demand for vaccines for children is increasing day by day because of the emergence of new virus variants. As a result, immunity is waning, and children are getting vulnerable to severe infectious diseases. Hence, parents must take a step forward to vaccinate their kids to reduce the risk of disease transmission.
Why Should COVID-19 Vaccines Be Given to Children?
Children have strong immunity and are less likely to suffer from severe COVID-19 symptoms. Some remain asymptomatic and recover from the infection without any treatment. However, numerous children have been diagnosed with severe viral symptoms. These symptoms have either turned fatal or led children to intensive care units. In addition, children with underlying medical conditions like kidney failure, heart disease, obesity, or asthma are more likely to develop infections. They are also at a higher risk of developing long COVID-19 symptoms. Long COVID-19 is a situation wherein the COVID-19 symptoms persist for several weeks or months. Some new studies suggest that there is a link between COVID-19 and multisystem inflammatory syndrome (MIS-C) in kids. It is a rare condition that can occur several weeks after a COVID-19 infection, resulting in inflammation of vital body structures, including the heart, lungs, skin, eyes, kidneys, or brain. In addition, it can cause multi-organ failure, shock, and even death in severe cases. Though it is a proven fact that children are less likely to develop severe COVID-19 symptoms, vaccines can further lower this risk. Studies report that vaccines provide protection against MIS-C too. Also, children who are vaccinated have fewer chances of getting a negative impact from COVID-19 on their education and their daily activities.
Which COVID-19 Vaccines Have Been Approved for Children Six Months or Older?
Children aged six months to 11 years need two doses of approved COVID-19 vaccines. Children are considered fully vaccinated two weeks after their second pediatric dose. Parents must not get worried as children between the ages of six months to five years. Hence, children from six months to 11 years of age will be vaccinated with Pfizer-BioNTech or Moderna COVID-19 vaccine. Those above 12 years of age are given the normal dose of the Pfizer vaccine. According to the American Academy of Pediatric guidelines, one vaccine product cannot be given with the other. The young child or baby will be administered two doses of the original vaccine followed by the third dose of an updated vaccine. Vaccines strengthen a child’s immune system and protect them from severe diseases.
Finally, the US (United States) FDA (Food and Drug Administration) has announced the approval of the updated bivalent Moderna and Pfizer-BioNTech COVID-19 vaccines for children between six months to five years of age. It has been named the bivalent vaccine because it includes two components. One of them has the original virus strain, and the other component is of the Omicron variant. The updated bivalent vaccine is to be used as a booster dose. The Moderna COVID-19 vaccine must be used as a single booster dose in children six months to five years of age two months after the completion of the monovalent series. The Pfizer COVID-19 vaccine must be used as a single booster dose in children five years of age or above, followed by primary vaccination with an authorized COVID-19 vaccine.
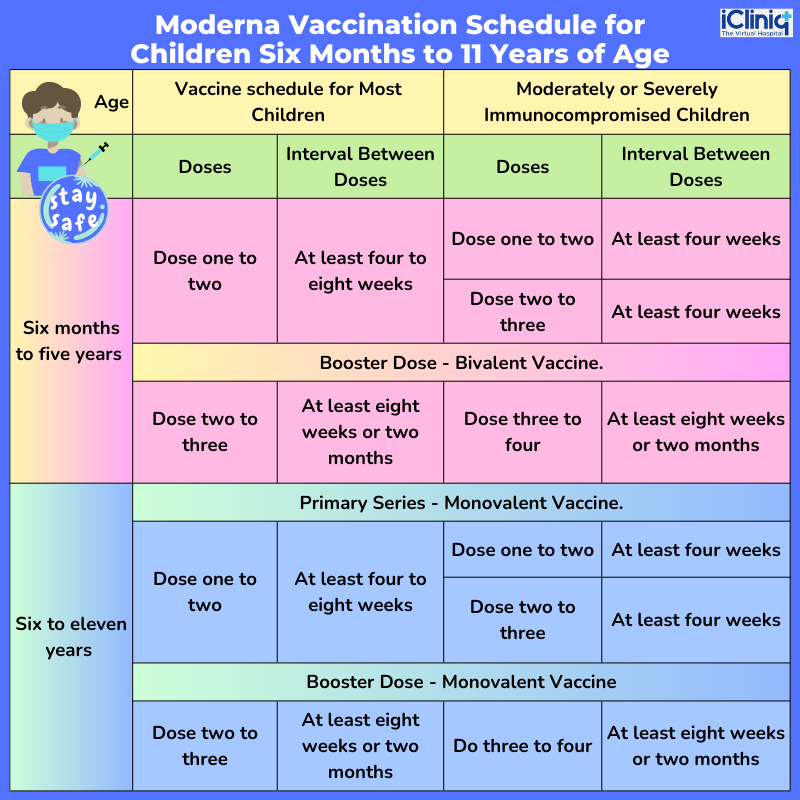
What Is the Interim COVID-19 Vaccination Schedule for Children Six Months of Age or Above?
The Moderna vaccination schedule for children six months to 11 years of age is listed below:
What Is the Important Information One Should Know About the Updated Bivalent Vaccine?
The parents must know the following about the updated bivalent vaccine:
-
Children from six months to five years of age who received the original or monovalent Moderna vaccine are eligible to get the single booster dose of the updated bivalent vaccine two months after the primary series.
-
Children six months to four years of age who have not initiated their three-dose primary series of the Pfizer-BioNTech COVID-19 vaccine or the third dose of the primary series can now be given the updated bivalent vaccine as the third dose in their primary series after two doses of original or monovalent vaccine.
-
Children from six months to four years of age who have already completed their three doses with the original or monovalent Pfizer-BioNTech COVID-19 vaccine are ineligible to receive the booster dose of the updated bivalent vaccine. This is because children under this age group will be protected against the serious outcomes of the Omicron variant.
-
Children who receive the updated bivalent vaccine will report side effects similar to the prior doses of monovalent vaccines.
What Are the Common Side Effects of COVID-19 Vaccines in Children?
The side effects of COVID-19 vaccines vary from one individual to the other. Some children have a little discomfort during their daily activities. However, these side effects usually subside in a few days. The following side effects can vary according to different age groups:
Children Six Months to Three Years of Age:
-
Pain in the legs or arms where the injection was given.
-
Irritability.
-
Swollen lymph nodes.
-
Reduced appetite.
Children Four to 17 Years of Age:
-
Pain.
-
Swelling.
-
Redness.
-
Tiredness.
-
Pain in the muscles or joints.
-
Chills.
-
Swollen lymph nodes.
Conclusion:
COVID-19 vaccines have played a crucial role in providing immunity against the disease. Though numerous vaccines are available in the market, a child must be given only the approved vaccine. Hence, parents must consult the doctor to know about the right vaccine.












