Introduction:
Asfotase alfa is the first and foremost enzyme replacement therapy indicated in patients suffering from infantile and juvenile-onset hypophosphatasia (HPP). The drug also treats the underlying cause of HPP-deficient tissue nonspecific alkaline phosphatase. Asfotase alfa is a unique drug because it is a first-in-class bone-targeted enzyme replacement therapy. Studies report that Asfotase alfa replaces the deficient alkaline phosphatase and reduces the elevated enzyme substrate levels. As a result, bone mineralization gets improved. Asfotase alfa also prevents serious skeletal and systemic patient morbidity and premature death of children due to hypophosphatasia. In simple terms, Asfotase alfa is a version or a type of alkaline phosphatase enzyme (ALP) and works as a replacement therapy for people suffering from ALP deficiency.
Drug Developmental History and Approval Timeline:
-
Asfotase alfa was developed and is currently manufactured by Alexion pharmaceuticals. The United States Food and Drug Administration (FDA) granted it the designation of an orphan drug in September 2008.
-
The safety and efficacy of the Asfotase alfa were evaluated in four open-label trials accompanied by supporting trials on 112 patients suffering from hypophosphatasia. These patients have been treated with Asfotase alfa for seven and a half years.
-
The trial results were miraculous as, the patients showed an overall survival rate of 94 % compared to those who were left untreated for prolonged periods.
-
Finally, the drug was approved by the United States FDA (Food and Drug Administration) on 23rd October 2015 for perinatal, infantile, and juvenile-onset hypophosphatasia.
General Information About Asfotase Alfa:
How Does Asfotase Alfa Work to Treat Hypophosphatasia?
Hypophosphatasia is a hereditary disorder that causes loss of tissue-nonspecific alkaline phosphatase enzyme. Asfotase alfa is an innovative bone-targeted enzyme replacement therapy that mainly targets the exact cause of hypophosphatasia. People suffering from hypophosphatasia have elevated levels of inorganic pyrophosphate, which blocks the growth of hydroxyapatite crystals. As a result, bone mineralization is inhibited, resulting in the accumulation of the bone matrix that is unmineralized. It manifests as rickets and bone deformities in infants and children. This is the time when Asfotase alfa comes into action. It replaces the missing enzyme because the drug contains a soluble glycoprotein comprising two identical polypeptide chains. Each contains an immunoglobulin and deca-aspartate peptide that works as a bone targeting domain.
What Makes Asfotase Alfa Unique?
Asfotase alfa is a unique drug because it directly targets the pathogenesis of hypophosphatasia. Before the implementation of Asfotase alfa recombinant enzyme replacement therapy, the treatment of hypophosphatasia was only limited to supportive care. The other interventions that were made to manage hypophosphatasia include the administration of parathyroid hormone, Prednisone, and plasma for several years. However, all these treatment measures failed to produce any long-term improvements. Next, the researchers tried the bone marrow transplantation procedure but failed to produce the desired results. These failures opened a new avenue for the development of Asfotase alfa. During the clinical trials, patients treated with Asfotase alfa showed 94 % clinical response against hypophosphatasia. Therefore, the drug was approved by the FDA and marked a revolution in managing hypophosphatasia.
What Are the Uses of Asfotase Alfa?
Asfotase alfa is the first and the only FDA-approved enzyme replacement therapy used to manage perinatal or infantile and juvenile-onset HPP. HPP is a rare condition diagnosed in only one out of 100,000 people. People suffering from this condition demonstrate the most severe perinatal and infantile forms. Asfotase alfa has been indicated for managing HPP in only those populations. The major advantage of the drug is that it replaces the enzyme and stimulates bone mineralization. In simple terms, Asfotase alfa slows down the rate of progression of bone malformation, eases the symptoms, and assists with mobility.
Dosage and Administration:
Dosage of Asfotase Alfa for Perinatal or Infantile-Onset Hypophosphatasia:
The recommended dosage regimen for Asfotase alfa for the management of perinatal or infantile-onset hypophosphatasia is 6 mg per kg per week.
The drug is mainly administered subcutaneously as either:
-
2 mg per kg three times weekly or
-
1 mg per kg six times weekly. The adverse reactions at the injection site might limit the tolerability of the six times per-week regimen of the drug.
The doctor might increase the dose of Asfotase alfa to 9 mg per kg if the patient does not demonstrate any improvement in the respiratory status, radiographic findings, growth, or lack of efficacy. In such a situation, the drug will be administered as 3 mg per kg three times per week.
Dosage of Asfotase Alfa for Juvenile-Onset Hypophosphatasia:
The recommended dosage of Asfotase alfa for the treatment of juvenile-onset hypophosphatasia is 6 mg per kg per week.
The drug is mostly administered subcutaneously as,
-
2 mg per kg three times per week.
-
1 mg per kg six times weekly. The infusion reactions at the injection site might limit the tolerability of the drug.
Administration of the Drug Based on the Patient’s Weight:
The drug is administered based on the patient’s weight.
The dosages of the drug as per the patient’s weight are mentioned in the tables below:
Weight-Based Drug Administration of 2 mg per Kg Three Times Weekly:
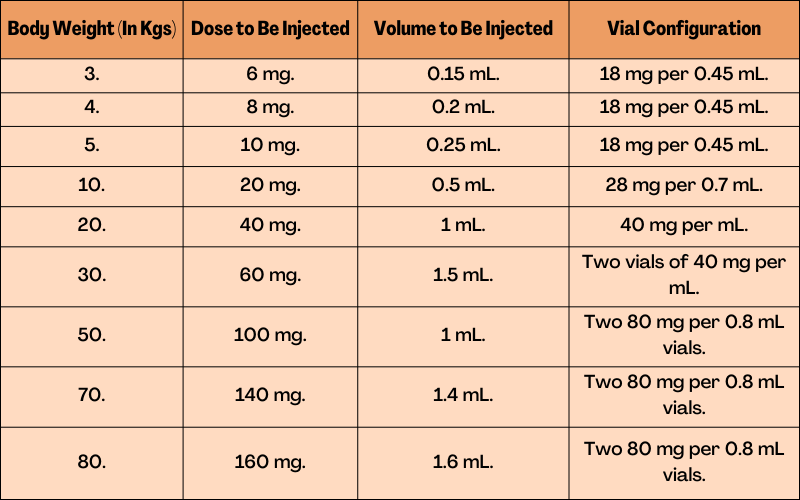
Weight-Based Drug Administration of 1 mg per kg Six Times Weekly:
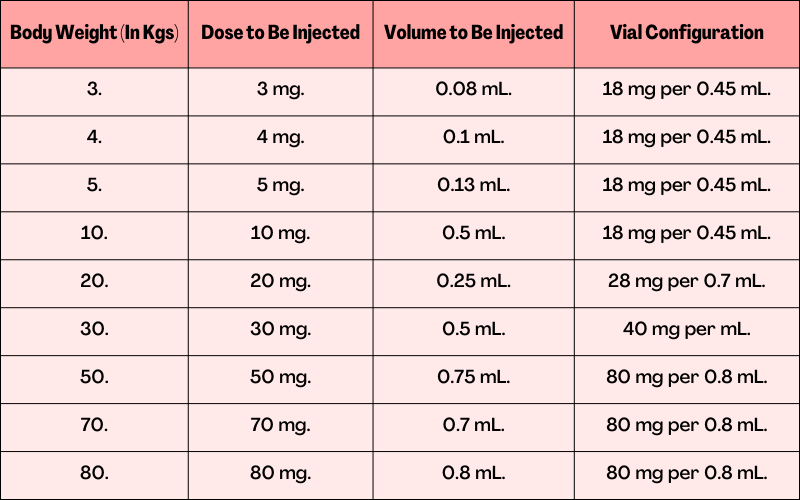
Weight-Based Drug Administration of 3 mg per kg Three Times Weekly (Only for Perinatal or Infantile-Onset Hpp):
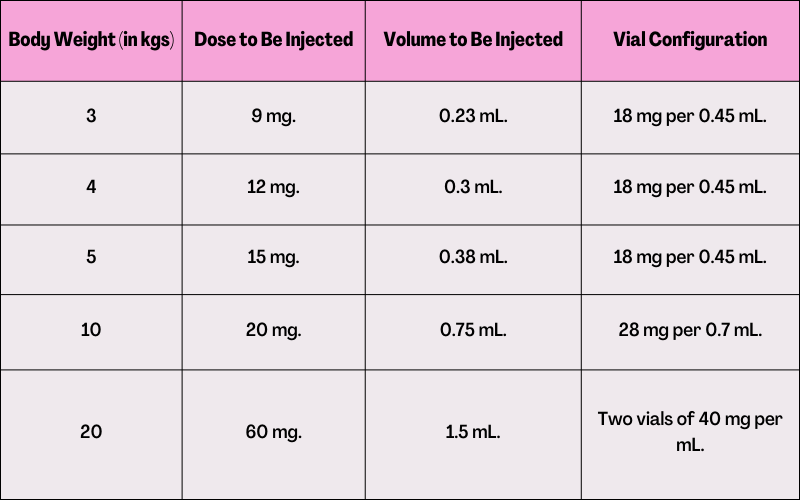
Information about Hypophosphatasia for Patients:
What Is Hypophosphatasia?
Hypophosphatasia is a rare inherited disorder characterized by the disrupted mineralization of bones and teeth. Mineralization refers to the procedure wherein the bones and teeth take up calcium and phosphorus to enhance their growth, strength, and hardness. If the mineralization procedure gets disturbed, the bones remain soft, and teeth might become loose, leading to tooth loss. Hypophosphatasia or Rathbun disease mainly occurs due to mutations in the genes. Normally, the body produces the ALP enzyme that stimulates the bone mineralization procedure. However, people suffering from hypophosphatasia have mutations in the genes that code for the ALP enzyme. As a result, the bones fail to mineralize, resulting in delayed growth and development of children and adults.
What Are the Signs and Symptoms of Hypophosphatasia?
The signs and symptoms of hypophosphatasia vary from one individual to the other. Sometimes, the symptoms can be mild, serious, or fatal, depending on the amount of ALP enzymes present in the body.
The signs and symptoms of HPP are listed below:
-
Curved arms and legs.
-
Failure of growth in babies.
-
Short stature.
-
Fused skull bones.
-
The wrist and ankle bones appear wider than normal.
-
Respiratory problems.
-
Soft, weak, and deformed bones.
-
Pain in the bones and joints.
-
Walking difficulties.
-
Bone fractures.
-
Severe arthritis.
For Patients:
Asfotase alfa is a prescription medication used to manage people with perinatal, infantile, and juvenile-onset hypophosphatasia. Studies report that Asfotase alfa is a soluble glycoprotein that contains polypeptide chains composed of 726 amino acids. The two chains are linked covalently by disulfide bonds. Asfotase alfa is a tissue non-specific enzyme replacement drug produced by recombinant DNA technology in the Chinese hamster ovary cells. The drug is mainly sterile, free of preservatives, nonpyrogenic, clear, or opalescent aqueous solution meant for subcutaneous administration. The specific activity of Asfotase alfa is 625 to 1250 units per mg. Specific activity basically refers to the amount of Asfotase alfa required to produce one micromol of p-nitrophenol from pNPP per minute at 37 degrees Celsius.
What Should the Patient Inform the Doctor Before Taking Asfotase Alfa?
Before taking Asfotase alfa, the patient must inform the doctor if he or she is:
-
Allergic to the drug or any of its ingredients. The patient must read the drug information leaflet carefully before consuming it.
-
Pregnant or planning to get pregnant in the future. However, it has not been known whether Asfotase alfa can harm the unborn baby.
-
Breastfeeding or planning to breastfeed the baby. Although no information is available on whether the drug passes from the mother’s milk to the baby or not. The lactating mother must consult the doctor before taking the drug.
-
Taking herbal supplements, prescriptions, and over-the-counter medications. The patient must inform the doctor about all the medications he is taking.
How Should the Patient Use Asfotase Alfa?
-
The patient must carefully read the “Instructions for use” provided with Asfotase alfa to know the right methods of using the drug.
-
The patient must Asfotase alfa exactly as the doctor tells him to.
-
The doctor will advise the patient on how much of the drug he needs to use and when it should be used.
-
The doctor might change the dose if required.
-
The patient must change the injection site after each injection. Avoid using the same injection site.
What Are Some of the Possible Side Effects of Asfotase Alfa?
Asfotase alfa can cause some of the following serious side effects that are listed below:
-
Allergic or Hypersensitivity Reactions - People consuming Asfotase alfa have presented with serious allergic or hypersensitivity reactions. The patient must stop taking the drug if such reactions occur and visit the nearby emergency room. The signs and symptoms of allergic reactions are listed below:
-
Difficulty in breathing.
-
Swollen eyes, lips, and tongue.
-
Hives.
-
Dizziness.
-
Nausea.
-
Itching of the tongue, lips, and throat.
-
-
Lipodystrophy - The thickening of the skin or formation of pits at the injection site is known as lipodystrophy. It might occur a few months after taking the drug.
-
Sometimes, calcium might build up in the eyes and the kidneys. The patient must undergo eye and kidney checkups before taking the drug.
Some of the common side effects of Asfotase alfa are listed below:
-
Red patches on the skin.
-
Bruising.
-
Change in skin color.
-
Pain.
-
Thinning.
-
Swelling.
-
Formation of pits and bumps.
General Information About the Safety and Effective Use of Asfotase Alfa:
Medications might be prescribed for purposes other than those mentioned in the patient information leaflet. However, the patient must refrain from using the drug for its not prescribed condition. Do not administer Asfotase alfa to others even if they have similar symptoms as the drug might turn harmful for them. The patient can consult the doctor to know more about Asfotase alfa.
For Doctors:
Description:
Asfotase alfa contains two identical polypeptide chains and is a soluble glycoprotein. There are 726 amino acids present in each polypeptide chain with a theoretical molecular mass of 161 kDa. Each polypeptide chain contains a catalytic domain of human-tissue nonspecific alkaline phosphatase (TNSALP), the human immunoglobulin G1 Fc domain, and a deca aspartate peptide. This deca aspartate peptide mainly works as a bone targeting domain. The TNSALP works as a metalloenzyme that catalyzes the hydrolysis of phosphomonoesters by releasing inorganic phosphate and alcohol. Asfotase alfa is slightly opalescent, colorless to slightly yellow, and comprises a few small translucent or white particles. The drug is supplied in a glass vial and is administered subcutaneously.
Indications and Usage:
Asfotase alfa is mainly indicated for the management of patients with perinatal or infantile and juvenile-onset hypophosphatasia.
Clinical Pharmacology:
Mechanism of Action:
Hypophosphatasia occurs due to the deficiency in the working of the TNSALP enzyme. As a result, the TNSALP substrates, including inorganic pyrophosphate levels, get elevated, resulting in the blockage of hydroxyapatite crystals. These crystals inhibit bone mineralization resulting in the accumulation of unmineralized bone matrix. As a result, infants and children suffer from rickets, osteomalacia, bone deformation, and muscle weakness. When the drug Asfotase alfa replaces the TNSALP enzymes, the enzyme-substrate levels are reduced, improving bone health.
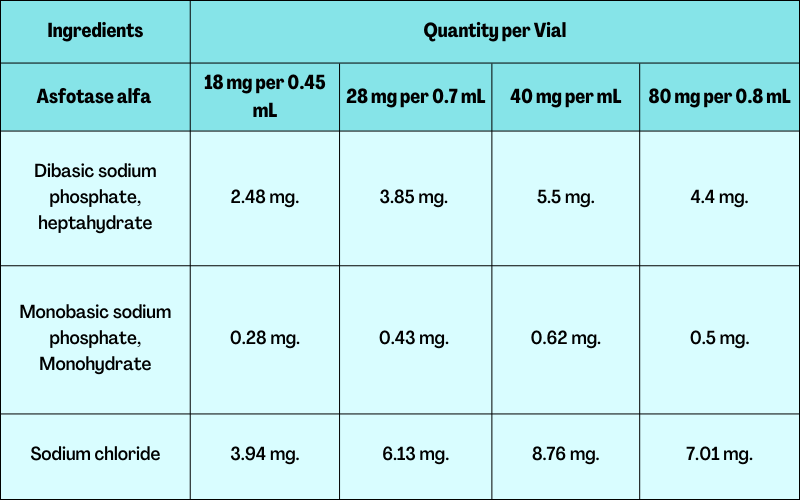
Composition of Asfotase Alfa:
Active Ingredients: Asfotase alfa.
Inactive Ingredients: Dibasic sodium phosphate, heptahydrate, monobasic sodium phosphate, monohydrate, and sodium chloride.
The table below describes the contents of the drug in detail:
Pharmacodynamics:
When people suffering from perinatal or infantile and juvenile-onset hypophosphatasia were treated with Asfotase alfa, reductions in the plasma TNSALP substrates, including inorganic pyrophosphate (PPi) and pyridoxal 5-phosphate (PLP) were observed after six to 12 weeks of treatment. However, reductions in the PPi and PLP levels were not correlated with the clinical outcomes. Bone biopsy data obtained from the patients with perinatal or infantile and juvenile HPP and treated with Asfotase alfa revealed a decrease in the osteoid volume and thickness, indicating improved bone mineralization.
Pharmacokinetics:
A dose proportionality across the dose range of 0.3 mg per kg to 3 mg per kg was observed in the pharmacokinetics when the analysis was done on 38 HPP patients. Patients taking the drug demonstrated steady-state exposure as early as three weeks after administering the first dose. The drug's elimination half-life was observed to be five days after the subcutaneous administration. Based on the population PK analysis, a weight-based dosing regimen was considered for Asfotase alfa because body weight is an integral factor for Asfotase alfa clearance. Patients taking Asfotase alfa demonstrated the formation of anti-drug antibodies resulting in reduced systemic exposure to the drug.
Non-clinical Toxicology:
Carcinogenesis, Mutagenesis, and Impairment of Fertility:
No long-term studies have been performed to evaluate the carcinogenic or mutagenic potential of Asfotase alfa. However, no adverse effects on fertility or reproductive potential were noted after Asfotase alfa 50 mg per kg per day was administered to animals three times weekly.
Dosage Form and Strength:
Asfotase alfa is always supplied as a sterile, preservative-free, nonpyrogenic, clear, slightly opalescent, and colorless to a slightly yellow color aqueous solution. The drug might also contain a few small translucent and white particles. The drug is available under the following dosages in a single-use vial:
-
18 mg per 0.45 mL.
-
28 mg per 0.7 mL.
-
40 mg per mL.
-
80 mg per 0.8 mL.
Contraindications:
Nothing is known about the contraindications of the drug.
Warnings and Precautions for the Drug:
-
Hypersensitivity Reaction - Severe allergic or hypersensitivity reactions have been reported in patients taking Asfotase alfa. During the clinical trial done on 99 patients, 1 % of them reported signs and symptoms related to anaphylaxis, including respiratory difficulties, nausea, dizziness, and periorbital edema. The reaction occurred a minute after the drug was administered but resolved without any medical treatment. The patient who exhibited hypersensitivity reactions resumed treatment with Asfotase alfa and received Diphenhydramine for an unspecified period. The patient subsequently continued the treatment with Asfotase alfa without taking any pre-medications. Other hypersensitivity reactions noted after taking the drug are listed below:
-
Vomiting.
-
Fever.
-
Chills.
-
Headache.
-
Flushing.
-
Irritability.
-
Skin erythema.
-
Rashes.
-
Pruritus.
-
Oral hypoesthesia.
-
If severe hypersensitivity reactions occur, the patient can discontinue the drug and consult the doctor for appropriate treatment. The doctor must consider the risk-benefit ratio before administering the drug to individual patients. If the drug has to be mandatorily administered, the patient must be carefully evaluated for the recurrence of signs and symptoms related to the hypersensitivity reactions.
-
Lipodystrophy - Localized lipodystrophy, lipohypertrophy, and lipoatrophy can occur at the injection site after several months in patients treated with Asfotase alfa. The patients must be instructed to follow the proper injection technique and rotate the injection sites.
-
Ectopic Calcifications - People suffering from HPP are more likely to develop ectopic calcifications. During the clinical trials with Asfotase alfa, 14 % of the patients reported ectopic calcification of the eye, including the cornea and conjunctiva. However, insufficient information was available to determine whether the reported side effects were due to the drug. No changes were observed in the vision or renal function due to the presence of ectopic calcifications. Therefore, the patient must undergo ophthalmic examinations and renal ultrasounds prior to the treatment with Asfotase alfa.
What Are the Adverse Reactions of Asfotase Alfa?
The following adverse reactions were noted in the patients during and after the clinical trial:
-
Erythema.
-
Discoloration.
-
Hypopigmentation.
-
Pain or tenderness.
-
Pruritus or itching.
-
Swelling.
-
Induration.
-
Macule.
-
Bruising.
-
Nodule.
-
Injection site hypertrophy.
-
Injection site atrophy.
-
Vomiting.
-
Hypersensitivity reactions.
Some of the less common adverse reactions are listed below:
-
Hypocalcemia.
-
Renal stones.
-
Chronic hepatitis.
-
Reduced vitamin B6.
Immunogenicity:
There is a potential for immunogenicity with all the therapeutic proteins. Anti-drug antibodies were detected in patients who received treatment with Asfotase alfa using the electrochemiluminescent immunoassay. The antibody samples obtained from the patients were tested for the presence of neutralizing antibodies. Out of the 98 patients enrolled for the trial, 78 % tested positive for anti-drug antibodies. However, the antibody detection method and the results vary according to the following factors:
-
Assay methodology.
-
Handling of the sample.
-
Sample collection timing.
-
Concomitant medications.
-
Underlying diseases.
Preparation of the Drug for Administration:
Note - Avoid using 80 mg per 0.8 mL vial of Asfotase alfa in children weighing less than 40 kg because the risk of systemic exposure is more with a higher concentration of the drug than with a lower concentration. Therefore, a lower exposure might not be adequate for the pediatric group.
-
Calculate the volume required for the prescribed dose based on the patient’s weight and the recommended drug dosage.
The following method can be used to calculate the patient's dose:
-
Total Drug Dose (mg) = Weight of the patient (kg) x prescribed dose (mg per kg).
-
Total Injection Volume (mL) = Total dose/concentration (40 mg per mL or 80 mg per mL).
-
Total Number of Vials = Total injection volume divided by the volume of the vial.
-
Calculate the exact frequency of weekly injections.
-
The patient’s weight must be rounded to the nearest kilogram while determining the dose.
-
When the volume of injection is more than 1 mL, the drug dosages must be split equally between the two syringes. Use a different injection site while administering the two syringes.
-
Carefully examine the solution in the vial for discoloration and particulate matter because Asfotase alfa is supplied as a sterile clear and a slightly opalescent liquid. A few translucent particles might be present in the drug. Discard the vial if it does show the actual appearance.
-
Assemble the injections. Asfotase alfa must be administered using the sterile disposable 1 mL syringes using the ½ inches injection needle between 25 and 29 gauge. If a dose larger than 1 mL is to be administered, the injection volume must be equally divided between the two 1 mL syringes. The patient must always use a new syringe and a new needle.
-
Remove the cap of the vial and prepare it aseptically. Insert the syringe into the vial to withdraw the prescribed amount of the drug.
-
Make sure that there are no air bubbles in the syringe and the dosage is correct.
Method of Administration of Asfotase Alfa:
-
Asfotase alfa must be administered within an hour after the removal of the vial from the refrigerator.
-
Change the injection site to reduce the risk of lipodystrophy. The injection sites can be the abdominal area, thigh, or groin.
-
Avoid administering the injections in areas that appear red, swollen, or inflamed.
-
Asfotase alfa must be injected subcutaneously in the predetermined site, and the injection must be disposed of carefully.
-
Asfotase alfa vials must be used only once. Discard the unused portion.
Instructions for the Use of Asfotase Alfa:
Asfotase Alfa (Injection, for Subcutaneous Use Vial)
Note - The patient must read the information booklet carefully before taking the drug and each time he gets the refill. Sometimes, new information might be available regarding the drug. The patient must always consult the doctor to stay updated regarding the drug information. The patient must avoid sharing his syringes or needles with anyone as he might have an infection or might infect others.
Supplies Needed for Asfotase Alfa Injection:
-
One or two vials of Asfotase alfa.
-
One or two sterile disposable 1 mL syringes with 25 to 29 Gauge ½ inch needles.
-
Two alcohol wipes.
-
One gauze or cotton balls.
-
A clean and flat surface like a table.
-
A sharp container for disposing of the used needles and syringes.
Preparing the Dose of Asfotase Alfa:
-
Prepare a tabletop or a clean surface.
-
The patient must collect all the supplies required for the Asfotase alfa injection.
-
The patient must wash his hands with soap and water.
-
Step 1 - Carefully evaluate the liquid in the Asfotase alfa vial. It should appear clear or slightly yellow and might contain a few white particles. Avoid using the vial if the liquid is discolored or comprises large particles or lumps. Discard the liquid and use a new vial.
-
Step 2 - The patient must use his thumb to remove the plastic cap from the Asfotase alfa vial.
-
Step 3 - Take out the needle from the package and place it at the top of the syringe. Push the needle downwards and twist it onto the syringe until the needle becomes tight.
-
Step 4 - Hold the syringe carefully. The needle must point upwards. Pull back the plunger until it reaches the marking for the prescribed dose.
-
Step 5 - Remove the cap straight from the needle. Avoid touching the needle and make sure the needle does not touch other surfaces.
-
Step 6 - Hold the drug vial firmly on a stable surface and push the needle into the vial through the rubber stopper.
-
Step 7 - Lift the vial upside down, keeping the needle intact. Now, the needle points towards the ceiling. Slowly push the plunger inwards.
-
Step 8 - Pull back the plunger slowly until the top of the plunger goes beyond the line marked for the prescribed dose. However, make sure that the needle tip still lies in the liquid. Do not pull out the needle from the vial. The patient must recheck the syringe to have the right dose of the drug.
-
Step 9 - Turn the drug vial upright and pull the syringe out of the vial's rubber stopper. Avoid touching the needle.
-
Step 10 - Hold the syringe so that the needle points upwards. Tap the barrel twice or thrice to remove the air bubbles.
-
Step 11 - Inject Asfotase alfa as mentioned by the doctor and use it within an hour after removing it from the refrigerator.
-
Step 12 - Choose the appropriate injection site. Asfotase alfa can be injected under the skin of the abdomen, upper arms, or upper legs. The patient must use a different injection site every time. Wipe the skin before taking the injection. The skin must be dry and free from inflammation, redness, or swelling.
-
Step 13 - The patient must pinch a small portion of the skin and hold the syringe at 90 degrees for injection. If a patient has fat, the syringe must be held at 45 degrees and then inserted into the skin.
-
Step 14 - Push the plunger at once to administer the dose.
-
Step 15 - Take the needle out of the skin. The patient must not recap the needle as it might lead to needle stick injury. The patient must press the injection site with a piece of gauze or alcohol wipes if he observes blood after removing the needle from the skin.
Disposal of the Used Syringes and Needles:
-
After use, the patient must put his needles in an FDA-cleared sharps disposal container. Avoid disposing of the loose needles and syringes in the household trash.
-
If the patient does not have the FDA-cleared sharps disposal container, he might use the household container that is:
-
Made of heavy-duty plastic.
-
Closed with a tight-fitting and puncture-resistant lid. The sharps must not come out of the disposal container.
-
Upright and remains stable during and after the use.
-
Leak proof.
-
Labeled to warn others of the hazardous material present inside the container.
-
-
The patient must follow the community guidelines after the sharps disposal container is full.
-
Avoid recycling the needles or syringes after they have been disposed of.
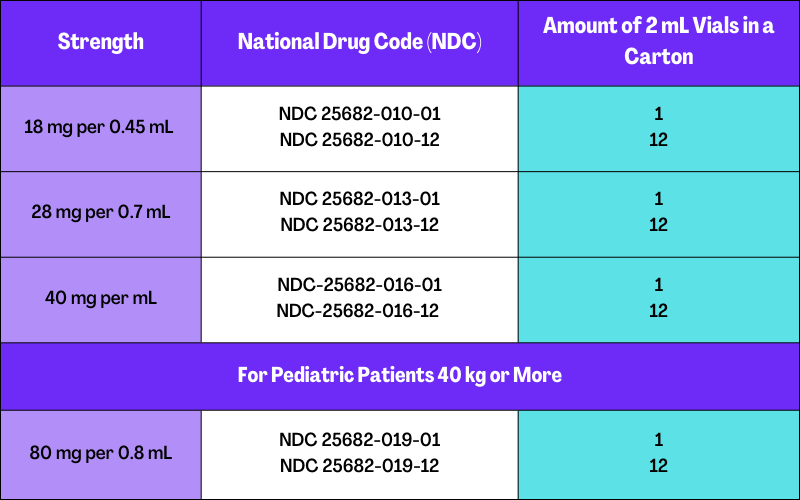
How Is Asfotase Alfa Supplied or Stored?
Asfotase alfa is available as a single-use vial in a carton containing 1 to 12 vials. The drug is sterile, nonpyrogenic, clear, slightly opalescent, and colorless to the slightly yellow-colored aqueous solution. The drug vials are available in the following strengths:
Storage of the Drug:
-
Asfotase alfa must be stored in the original carton till the time of use. The drug must be refrigerated at 2 to 8 degrees Celsius and protected from direct sunlight.
-
After the drug has been removed from the refrigerator, it must be administered within an hour.
-
Do not use the drug beyond the expiration date.
-
Avoid freezing or shaking the drug.
Additional Information About Asfotase Alfa:
Use in a Specific Population:
Pregnancy:
No studies have been conducted on humans to evaluate the effects of Asfotase alfa on a pregnant female. During the animal studies, no clinically significant evidence of embryotoxicity, fetotoxicity, or teratogenicity has been noted.
Lactation:
There is no information available regarding the presence of Asfotase alfa in human milk or a breastfed infant. Therefore, the health benefits of breastfeeding must be considered with the mother’s need for Asfotase alfa.
Pediatric Patients:
The safety and efficacy of Asfotase alfa have been known with the help of four prospective open-label clinical trials done on 99 adult and pediatric patients.
Geriatric Population:
As the patients above 65 were not enrolled in the clinical trial, no information is available regarding the safety and effectiveness of the drug.
Clinical Trial:
Perinatal or Infantile-Onset HPP:
Study 1 - A 24-week prospective single-arm trial was done on 11 patients suffering from severe perinatal or infantile-onset HPP. The patients also had rachitic chest deformity, failure to thrive, and vitamin B6-dependent seizures. The patients received Asfotase alfa 3 mg per kg per week for the first month. The dose was subsequently increased to 9 mg per kg per week.
Study 2 - An open-label study was done on 59 patients who received Asfotase alfa at 6 mg per kg per week for the first four weeks.
Trial Results: The trial results demonstrated that 97 % of the newborns and infants diagnosed with HPP showed clinically significant survival rates compared to the 42 % of patients who received historical treatment.
Juvenile-Onset HPP:
A 24-week prospective open-label trial was done on eight randomized patients who received Asfotase alfa 6 mg per week or 9 mg per week. The dosing regimen of the patient was changed to 3 mg per kg per week in the extension phase.
Trial Results:
Patients with juvenile onset-HPP showed significant improvements in the skeletal manifestations of HPP at 24 weeks. After 54 months, it was noted that 100 % of the patients responded to the treatment with Asfotase alfa.












