Introduction:
Baloxavir marboxil is a cap-dependent endonuclease inhibitor. It is a prodrug that releases biologically active baloxavir acid. This drug works against the two types of influenza viruses that cause infections in humans, influenza A and B. Baloxavir marboxil is a revolutionary drug in treating influenza infection because its mechanism of action is different from the other drugs that were used to treat influenza. The older drugs inhibit the virus by blocking the viral enzyme called neuraminidase. In contrast, Baloxivir marboxil interferes with the ability of the influenza virus to multiply. It inhibits the subunit of viral polymerase, an enzyme responsible for influenza virus replication.
Drug Approval History and Timeline:
-
Baloxavir marboxil was widely recognized and launched in the market for medical use in the United States and Japan in 2018. It was developed as a prodrug strategy, and the drug released the active agent baloxavir acid after its metabolism.
-
The drug was developed for the market by a Japanese pharmaceutical company named Shionogi Co. and Switzerland-based Roche AG.
-
The drug was first approved by the US food and drug administration (FDA) on 24th October 2018 for the treatment of acute uncomplicated influenza in people 12 years of age or above who have been symptomatic for no more than 48 hours.
-
In October 2019, the FDA updated the indications of the drug, which states that it could be used for the treatment of uncomplicated acute influenza in people above 12 years of age or older at the risk of influenza complications.
-
Finally, in November 2020, the FDA approved another approved indication of the drug, which stated that it could be used for the post-exposure prevention of influenza for people 12 years of age or above after contacting the individual who had the flu.
General Information About Baloxavir Marboxil:
How Does Baloxavir Marboxil Work to Treat Influenza?
Influenza or flu is a viral infection that attacks the respiratory system, including the nose, lungs, and throat. It has been noted that influenza or flu viruses use the protein called endonuclease to replicate or make several copies of themselves. Baloxavir marboxil works by inhibiting this protein and stops the virus from replicating. In other words, the drug inhibits the process of cap snatching, by which the influenza virus derives short and capped primers from the RNA of the host cells. As a result, the drug allows the immune system to fight off the infections and helps people recover from the flu symptoms about a day faster than they would typically recover without the treatment. Flu or influenza virus strains change yearly, so different drugs must be available to treat them. Otherwise, the virus becomes resistant to the drug's mechanism of action.
What Are the Uses of Baloxavir Marboxil?
Baloxavir marboxil is specifically used to treat the symptoms of influenza A and B. As per the study reports, this drug reduces the duration of flu symptoms by about a day. It is used in children above 12 years to treat the following:
-
Influenza - Baloxavir marboxil is used to treat flu or influenza that does not require treatment in the hospital. It is mainly used for people who have had symptoms for up to 48 hours and are either:
-
Healthy and do not present with any complications or
-
At a higher risk of developing complications of flu such as pneumonia.
-
-
Prevention of Influenza - Baloxavir marboxil has been approved to prevent flu in people who have had contact with the patient infected with flu.
How Long Does the Patient Remain Contagious After Taking Baloxavir Marboxil?
Baloxavir marboxil helps the body fight the influenza virus or infection. However, the drug does not stop one from being contagious. The influenza virus is highly contagious and can be easily transferred to patients through the droplets they breathe out while coughing, sneezing, and talking. An infected person can transmit the influenza virus to a healthy person for five to seven days even after taking the drug. Some patients have a weak immune system and continue transferring the virus for more than five to seven days. However, the advantage of Baloxavir marboxil is that it starts working about four hours after taking the first dose. Most patients feel alright about two days after taking the drug.
Dosage and Administration:
Dosage and Administration Overview:
Baloxavir marboxil is available in two dosage forms:
-
Baloxavir marboxil tablets.
-
Baloxavir Marboxil Oral Suspension - It is a granule formulation intended for patients who find it difficult or cannot swallow tablets or those who need internal administration.
Baloxavir marboxil should be taken immediately after the onset of the influenza symptoms or exposure to influenza. The drug can be taken with or without food but should not be taken with the following:
-
Dairy products.
-
Calcium-fortified beverages.
-
Polyvalent cation containing laxatives.
-
Antacids.
-
Oral supplements (calcium, iron, selenium, magnesium, and zinc).
Recommended Dosage:
Management of Acute Uncomplicated Influenza or Post-exposure Prophylaxis in Adults and Adolescents (12 Years of Age or Above):
Baloxavir marboxil must be taken as a single dose as soon as possible or within 48 hours of the onset of influenza symptoms for the treatment of uncomplicated acute influenza. The recommended dosage of Baloxavir marboxil as tablets and oral suspension is listed in the table below:

Information About Influenza Infection for Patients (Baloxavir Marboxil Tablets and Oral Suspension):
What Is Influenza?
Influenza, also known as grippe or flu, is a contagious respiratory illness caused by the influenza virus. The disease affects the nose, throat, and lungs and can cause mild to severe illness. The best way to prevent influenza is by getting a vaccine annually. The influenza viruses circulate throughout the world and represent a year-round burden. Most patients have a fever and recover from the symptoms in about a week without requiring medical care and attention. However, influenza can cause severe illnesses among high-risk individuals, including the very young, pregnant females, elderly, health care workers, and those suffering from debilitating medical diseases.
What Are the Signs and Symptoms of Influenza?
The signs and symptoms of influenza are listed below:
-
Fever.
-
Muscle aches.
-
Chills and sweats.
-
Headache.
-
Dry and persistent cough.
-
Tiredness.
-
Weakness.
-
Nasal congestion.
-
Pain in the eyes.
-
Vomiting.
Information About Baloxavir Marboxil for Patients:
Baloxavir is a prescription medication used as a therapeutic agent against influenza. It is specifically used for patients 12 years of age or above for the management of influenza. The drug comes in tablets or granules that can be converted into a solution. Baloxavir marboxil is a prodrug and is taken orally. When the drug reaches the gastrointestinal tract, the marboxil component reveals the active drug, baloxavir acid. The biggest advantage of Baloxavir marboxil over the other drugs is that a single dose effectively reduces flu-like symptoms. An important point to be noted about this drug is that 2.2 % of the patients in the phase II trials and 10 % of the patients in the phase III trials had developed resistance to the drug. Therefore, there is a continuous demand for new drugs for flu and influenza because the virus keeps changing its strains. It is known if Baloxavir marbexil is effective in children less than 12 years of age. Baloxavir marboxil does not treat or prevent illnesses caused by infections other than the influenza virus. In addition, the drug does not prevent bacterial infections that might occur with flu.
What Important Things Must the Patient Inform the Doctor Before Taking Baloxavir Marboxil?
Before taking Baloxavir marboxil, the patient must inform the doctor if he or she is:
-
Is pregnant or planning to become pregnant. However, nothing has been known about the safety and effectiveness of the drug in pregnant females.
-
Is a lactating mother and is planning to breastfeed the baby. It has not been known if the drug can pass into breast milk.
-
Is allergic to Baloxavir marboxil and its other ingredients. The patient must know the composition of the drug before taking it to prevent allergic reactions.
-
Taking antacids or laxatives containing aluminum, calcium, magnesium, potassium, iron, and vitamin or mineral supplements.
-
Taking prescription or over-the-counter drugs, vitamins, and some other herbal supplements. The doctor might alter the dosage of the drug and will carefully monitor the patient for side effects.
-
Going to receive the live flu vaccine after taking Baloxavir marboxil.
How Should the Patient Take Baloxavir Marboxil?
-
The patient must take the drug as directed by their doctor or pharmacist.
-
The doctor might prescribe the following:
-
Baloxavir marboxil tablets that are to be taken at the same time as a single dose or
-
Baloxavir marboxil oral suspension comes with a measuring device, mainly an oral syringe or a measuring cup to be taken as a single dose.
-
-
Sometimes, the drug might be given through a feeding tube. In such situations, the patient must follow the doctor’s instructions for giving Baloxavir marboxil through the feeding tube.
-
The drug can be taken with or without food.
-
The patient must not take Baloxavir marboxil with dairy products, calcium-fortified beverages, laxatives, antacids, and oral supplements containing iron, selenium, calcium, magnesium, and zinc.
-
If the patient overdosed on Barloxavir marboxil, he could visit the nearby emergency room for treatment.
Baloxavir Marboxil for Oral Suspension:
-
The pharmacist will mix the drug for oral suspension before giving it to the patient. However, the patient can contact his pharmacist if the Baloxavir marboxil was not given as a liquid solution or a measuring device was not provided.
-
Take the drug before the expiration date and time mentioned by the pharmacist on the drug bottle.
-
Avoid taking Baloxavir marboxil if the date and time of the drug have passed. Discard the bottle and purchase a new one.
-
The patient might require more than one bottle for the total prescribed dose of Baloxavir marboxil.
How Should Balaxovir Marboxil Be Given as Oral Suspension?
-
Step 1 - Swirl the Baloxavir marboxil oral suspension bottle before each use, but avoid shaking the bottle.
-
Step 2 - Push the child-resistant bottle cap downwards to open it. Twist the cap in the direction of the arrow.
-
Step 3 - Use the measuring device provided by the pharmacist to measure the oral suspension so that the patient does not get more than the prescribed dose of the drug.
-
Step 4 - The patient must sit upright while taking Baloxavir marboxil.
-
Do not administer the drug when the patient is lying down.
-
Avoid mixing the drug with soft foods or other medications.
-
-
Step 5 - Give the full content of the measuring device as the patient might need more than one bottle of the drug.
-
Step 6 - Close the bottle and discard the remaining oral suspension and the measuring device.
What Are Some of the Possible Side Effects of Baloxavir Marboxil?
Baloxavir marboxil might cause the following side effects in the patient:
-
Allergic Reactions - The patient must seek emergency medical help if he observes the following side effects:
-
Respiratory difficulties.
-
Swelling of the face, throat, and mouth.
-
Hives and blisters.
-
Dizziness.
-
Lightheadedness.
-
-
The most common side effects of Baloxavir marboxil in adults and adolescents suffering from influenza are listed below:
-
Diarrhea.
-
Sinusitis.
-
Bronchitis.
-
Nausea.
-
Note - Baloxavir marboxil is ineffective against other infections accompanied by influenza. Different treatment options can manage other infections that occur along with flu. The patient must inform the doctor if they feel worse or develop other symptoms during or after the treatment with Baloxavir marboxil.
How Should the Patient Store Baloxavir Marboxil?
Baloxavir Marboxil Tablets:
-
Store the tablets at room temperature between 68 to 77 degrees Fahrenheit.
-
Store the Baloxavir marboxil tablets in blister packages only.
Baloxavir Marboxil for Oral Suspension:
-
Store the oral suspension bottle at room temperature between 68 to 77 degrees Fahrenheit.
-
Keep Baloxavir marboxil for oral suspension in the original container. Use the bottle before the expiration date and time mentioned on it.
-
Throw away the unused bottles after the expiration date and time.
-
Keep the oral suspension bottle out of reach of children.
General Information About the Safety and Effectiveness of Baloxavir Marboxil:
Sometimes, the doctor might prescribe the drug for purposes other than those mentioned in the patient information leaflet. However, the patient must refrain from using Baloxavir marboxil for the condition it was not prescribed. Avoid giving the drug to other people even if they have similar symptoms because the drug might harm them. The patient can ask the pharmacist or doctor about the information written for doctors and healthcare professionals.
Information for Doctors:
Description of Baloxavir Marboxil:
Baloxavir marboxil is an antiviral polymerase acidic (PA) endonuclease inhibitor. The active component of the drug is Baloxavir marboxil. The chemical formula of the drug is ({(12aR)-12-[(11S)-7,8-Difluoro-6,11-dihydrodibenzo[b,e]thiepin-11-yl]-6,8-dioxo-3,4,6,8,12,12a-hexahydro1H-[1,4] oxazino [3,4-c] pyrido [2,1-f][1,2,4] triazin-7-yl}oxy) methyl methyl carbonate. The empirical formula of the drugs is C27H23 F2 N3 O7 S. The molecular mass of Baloxavir marboxil is 571.55 grams per mole, and the partition coefficient is (log P) of 2.26. The drug is freely soluble in dimethylsulfoxide, soluble in acetonitrile, slightly soluble in methanol and ethanol, and practically insoluble in water. Baloxavir marboxil is supplied both as tablets and oral suspensions. The tablets are white to light yellow and film-coated for oral administration. Baloxavir marboxil for oral suspension is supplied as white to light yellow granules in an amber glass bottle. Each bottle comprises 40 mg of Baloxavir marboxil. The granules must be constituted using 20 ml of sterile water or drinking water to obtain a 2 mg per ml suspension having a strawberry flavor.
Clinical Pharmacology:
Mechanism of Action:
Baloxavir marboxil is an antiviral and a pro drug with activity against the influenza virus. It is converted into its active form, Baloxavir, by hydrolysis. The active form of this drug exerts anti-influenza viral activities. The drug inhibits the endonuclease activity of PA protein, which is an influenza-virus-specific enzyme. This enzyme is a part of the viral RNA polymerase complex and is required for viral gene transcription. As a result, the replication of the influenza virus is inhibited by the drug. In addition, viruses that showed reduced susceptibility to Baloxavir marboxil have amino acid substitutions in the PA protein.
Antiviral Activity:
An MDCK cell-based plaque reduction assay was done to evaluate the antiviral activity of the drug against laboratory strains and clinical isolates of influenza A and B viruses. As per the trial results, the median 50 % effective concentration values were 0.73 nM for H1N1 strains and 5.97 nM for type B strains. The 90 % effective concentration values of Baloxavir marboxil against avian subtypes were in the range of 0.80 to 3.16 nM. Nothing has been known about antiviral activity in humans.
Resistance:
The influenza A virus isolates having reduced susceptibility to Baloxavir marboxil were selected for cell culture in the presence of increasing concentrations of Baloxavir. During the clinical studies, 4.5 % of the patients with influenza A virus had reduced susceptibility to Baloxavir marboxil.
Cross-Resistance:
Cross-resistance between neuraminidase inhibitors and Baloxavir marboxil or between M2 proton pump inhibitors and Baloxavir could not be expected; these drugs work against different viral proteins. Baloxavir marboxil is effective against NA inhibitor-resistant strains, including A/H1N1 and A/H5N1 viruses. Other drugs are effective against these strains, but no clinical relevance of phenotypic cross-resistance evaluations has been established.
Immune Response:
Nothing has been known about the interaction of Baloxavir marboxil with influenza vaccines.
Composition of Baloxavir Marboxil:
Active ingredient - Balaxovir marboxil.
Baloxavir Marboxil Tablets: Inactive Ingredients - Croscarmellose sodium, hypromellose, lactose monohydrate, microcrystalline cellulose, povidone, sodium stearyl fumarate, talc, and titanium dioxide.
Baloxavir Marboxil Oral Suspension: Inactive Ingredients - Colloidal silicon dioxide, hypromellose, maltitol, mannitol, povidone K25, sodium chloride, strawberry flavor, sucralose, and talc.
Pharmacodynamics:
Cardiac Electrophysiology:
Baloxavir marboxil was given at twice the prescribed dose to evaluate the cardiac electrophysiology, but the drug did not prolong the QT interval.
Exposure-Response Relationship:
When Baloxavir marboxil was administered according to the weight, that is, 40 mg in patients of 40 to 80 kg and 80 mg in patients weighing 80 kg, no difference in the exposure-response relationship was observed.
Pharmacokinetics:
Baloxavir marboxil is a prodrug that gets converted to its active metabolite after oral administration. The pharmacokinetic properties of the drug are listed in the table below:
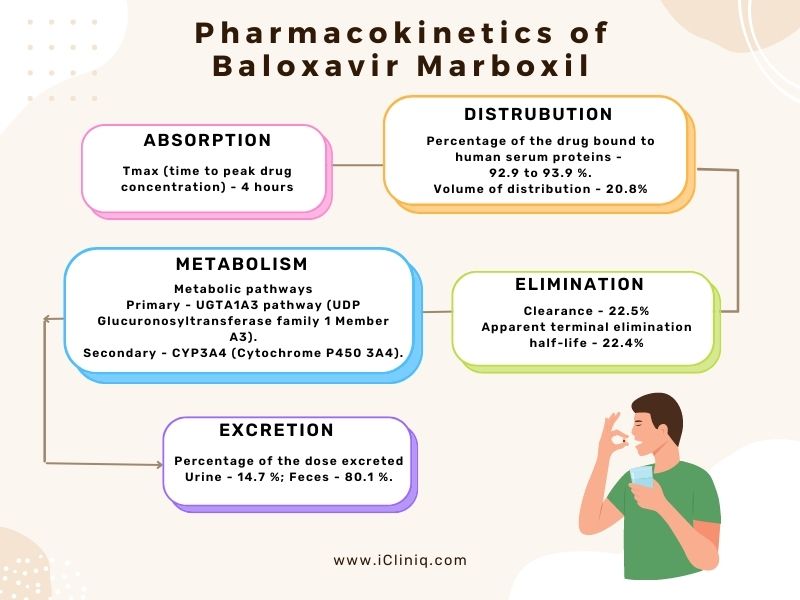
Body Weight:
The exposure to Baloxavir marboxil decreases as the body weight increases. However, no clinically significant differences were observed in the body weight after administering the recommended dose of the drug.
Indications and Usage:
- Treatment of Influenza:
Baloxavir marboxil is indicated for treating acute uncomplicated influenza in patients 12 years of age or above who have been symptomatic for no more than 48 hours and are otherwise healthy or at a higher risk of developing influenza-related complications.
- Post-exposure Prophylaxis of Influenza:
Baloxavoir marboxil is specifically indicated for post-exposure prophylaxis of influenza in patients 12 years of age or above and who had contact with an individual who has influenza.
Limitations of Use:
The influenza virus strains change over time according to the factors like virus type or subtype, the emergence of resistance, and change in viral virulence. These factors tend to diminish the clinical benefits of antiviral drugs. Therefore, the patient must refer to the information available on the drug susceptibility patterns for circulating influenza virus strains when deciding whether to use Baloxavir marboxil or not.
Dosage Forms and Strength:
Baloxavir Marboxil Tablets:
Baloxavir marboxil 20 mg tablets are white to yellow, oblong-shaped, and film-coated tablets, which are debossed with 772 on one side and 20 on the other side.
Baloxavir marboxil 40 mg tablets are white to yellow, oblong-shaped, and film-coated tablets, which are debossed with BXM40 on one side.
Baloxavir Marboxil for Oral Suspension:
Baloxavir marboxil for oral suspension contains 40 mg per 20 ml or 2 mg per ml of the drug after constitution with sterile or drinking water. As the drug granules are white to yellow, the constituted drug solution is a white to yellow colored opaque suspension with strawberry flavor.
Contraindications:
The drug is contraindicated in patients with a history of hypersensitivity to Baloxavir marboxil or any of its ingredients. This is because serious allergic reactions like anaphylaxis, urticaria, angioedema, and erythema multiforme have been noted.
Warnings and Precautions:
Hypersensitivity:
Postmarketing experience with the drug have reported that patients presented with side effects like angioedema, urticaria, anaphylaxis, and erythema multiforme. Appropriate treatment must be given if the patient presents with allergic reactions. The use of Baloxavir morbexil is contraindicated in patients showing hypersensitivity reactions.
Bacterial Infections:
Nothing has been known about the efficacy of the drug Baloxavir marboxil against the illness caused by pathogens other than the influenza virus. Serious bacterial infections might initiate or coexist with influenza-like symptoms, but Baloxavir marboxil is ineffective against these complications and infections. Therefore, the doctors must be aware of the potentially serious complications and treat them appropriately.
Adverse Reactions Based on the Clinical Trial Experience:
Clinical trials are conducted under various conditions, so the drug's adverse reactions cannot be compared to those seen in routine practice.
Treatment of Acute Uncomplicated Influenza:
Three placebo-controlled trials were done on 1640 adult and adolescent patients to evaluate the safety and efficacy of the drug. The trial reports indicated that 1 % of the patients presented with the following adverse reactions:
-
Diarrhea.
-
Nausea.
-
Headache.
Post-marketing Experience:
Some adverse reactions were reported from a population of uncertain size, so it is impossible to rely on the frequency of these adverse reactions. However, the following adverse reactions have been identified during post-marketing use of Baloxavir marboxil:
-
Disorders of the Immune System - Anaphylactic reactions, anaphylactic shock, hypersensitivity reactions, and angioedema.
-
Skin and Subcutaneous Tissue Disorders - Skin rashes, urticaria, and erythema multiforme.
-
Gastrointestinal Disorders - Vomiting, melena, colitis, and hematochezia.
-
Psychiatric Disorders - Delirium, abnormal behavior, and hallucinations.
Drug Interactions:
Baloxavir might form a chelate with polyvalent cations like calcium, magnesium, and aluminum. If the drug is coadministered with polyvalent cation-containing products, the efficacy of Baloxavir marboxil might get reduced. Avoid taking the drug with dairy products, calcium-fortified beverages, polyvalent cation-containing laxatives, antacids, or oral supplements.
Vaccines:
Nothing has been known about the concurrent use of Baloxavir marboxil and intranasal live attenuated influenza vaccine (LAIV). However, concurrent administration of antiviral drugs might inhibit the viral replication of LAIV and decrease its effectiveness.
Clinical Studies on Drug Interactions:
No clinically significant changes were observed in the pharmacokinetics of Baloxavir marboxil or its active metabolite when the drug was co-administered with the following:
-
Itraconazole (combined strong CYP3A and P-gp inhibitor).
-
Probenecid (UGT inhibitor).
-
Oseltamivir.
-
Midazolam (CYP3A4 substrate).
-
Digoxin (P-gp substrate).
-
Rosuvastatin.
Antacids:
The following antacids must not be taken with Baloxavir marboxil:
-
Calcium carbonate.
-
Aluminum hydroxide.
-
Magnesium hydroxide.
-
Bismuth sub salicylate.
-
Magnesium sulfate.
-
Citric acid.
-
Sodium hydrogen carbonate.
Laxatives:
The following laxatives must not be taken with Baloxavir marboxil:
-
Milk of magnesia.
-
Andrews original salts.
-
Epsom salt.
-
Citroma.
Non-clinical Toxicology:
Carcinogenesis:
Studies have not been performed to evaluate the carcinogenesis of Baloxavir marboxil.
Mutagenesis:
During the in vitro and in vivo genotoxicity assays, it was observed that Baloxavir marboxil and its metabolite Baloxavir were not mutagenic.
Impairment of Fertility:
Nothing has been known about the effect of the drug on fertility in humans. However, during the animal studies, Baloxavir marboxil at 20, 200, or 1000 mg/kg/day was administered to female rats for two weeks during and before mating. Male rats were dosed for four weeks before and during mating, but the drug did not impair fertility.
Preparation of Baloxavir Marboxil Oral Suspension:
Before giving the drug to the patient, the doctor must constitute Balovir marboxil for oral suspension with 20 ml of sterile water or drinking water. After the constitution is complete, each bottle of Baloxavir marboxil contains 40 mg per 20 ml or 2 mg per ml of the drug. Hence, this dosage form of the drug can be used for oral and enteral use.
Constituting Baloxavir Marboxil for Oral Suspension:
The suspension must be prepared at the time of dispensing. The oral suspension must be administered within ten hours after constitution because the product does not contain a preservative. Follow the below-mentioned steps:
-
Gently tap the bottom of the bottle as it helps loosen the granules.
-
Constitute Baloxavir marboxil for oral suspension with 20 ml of distilled or sterile water.
-
Swirl the suspension to ensure that the granules are evenly suspended, but do not shake the bottle.
-
Mention the expiration date and time on the bottle to avoid confusion.
Important Information for the Doctors:
-
Make sure that the patient has a measuring device, that is a measuring cup or an oral syringe, before taking the prescribed dose of the drug.
-
For enteral administration, use an enteral syringe to draw up the oral suspension and flush with 1 ml water before and after enteral administration.
-
The patient must be instructed that he might require more than one bottle for the total prescribed dose of Baloxavir marboxil.
Overdosage:
Suppose the patient had an overdose of Baloxavir marboxil. In that case, the treatment should mainly include general supportive measures like monitoring the vital signs and observation of the patient's clinical status. No specific antidote is available for overdose with Baloxavir marboxil. Moreover, as the drug has high serum protein binding, it is unlikely to be removed by dialysis.
How Is the Drug Supplied?
-
The drug is supplied as 20 mg white to light yellow, oblong-shaped, film-coated tablets available as:
-
2 x 20 mg tablets are present in one blister card in secondary packaging: NDC 50242-828-02.
-
-
The drug is supplied as 40 mg white to light yellow, oblong-shaped, film-coated tablets available as:
-
2 x 40 mg tablets are present in one blister card in secondary packaging: NDC 50242-860-02.
-
Storage of the Drug:
Baloxavir marboxil must be stored in the blister package at 20 to 25 degrees Celsius. Excursions are permitted to 15 to 30 degrees Celsius.
Baloxavir Marboxil for Oral Suspension:
The drug is supplied in an amber-colored glass bottle with a child-resistant cap at the dose of 40 mg per 20 ml. White to yellow-colored granules are constituted with distilled water or sterile water. The usable volume of the drug is 20 ml which is equivalent to 40 mg of Baloxavir marboxil.
Handling:
The product does not contain any preservatives, so it must be administered within 10 hours after constitution.
Some More Facts About Baloxavir Marboxil:
Use in Specific Populations:
Pregnancy:
When the controlled studies were done related to Baloxavir marboxil, nothing was known about the adverse effects of the drug in pregnant females. However, pregnant women suffering from influenza are at a higher risk of severe complications, including maternal death, stillbirth, birth defects, preterm delivery, and low birth weight babies. However, in animal reproduction studies, no adverse effects related to pregnancy were noted after the oral administration of Baloxavir marboxil.
Lactation:
Nothing has been known about the presence of Baloxavir marboxil in human milk, its effects on breastfed infants, and the effects on milk production. However, Baloxavir and its metabolites were discovered in the milk of lactating rats. Therefore, the doctors must consider the developmental and health benefits of breastfeeding along with the mother’s clinical need for Baloxavir marboxil. Also, the potential side effects of the drug on the breastfed child must be noted.
Pediatric Use:
Acute Uncomplicated Influenza:
Two randomized, double-blind controlled trials done on patients 12 years of age and above weighing 40 kg support that the drug is safe and effective for use in adults and adolescents. However, nothing has been known about the safety and efficacy of the drug in children below 12 years of age.
Post-exposure Prophylaxis Influenza:
A randomized, double-blind control trial done in Japan confirmed that the drug is safe and can be effectively used in children 12 years of age or above for the post-exposure prophylaxis of influenza.
Geriatric Use:
A double-blind trial was done in 730 patients, out of which 209 patients were above 65, to evaluate the safety and effectiveness of the drug. The trial results confirmed that adults above 65 years of age who received Baloxavir marboxil during the trial showed improvement of the influenza symptoms in less time than the ones who received a placebo. Hence, the drug has been declared safe for the geriatric population.
Clinical Studies:
Treatment of Acute Uncomplicated Influenza:
Adults and Adolescents (12 Years and Older):
Two randomized double-blinded clinical trials were done to evaluate the safety and efficacy of Baloxavir marboxil in patients with acute uncomplicated influenza.
Trial 1:
A placebo-controlled phase two trial was done on 400 patients aged 20 to 64 years in Japan.
Trial 2:
A phase three randomized, double-blind, active, and placebo-controlled trial was done in 1436 patients having symptoms of influenza.
Note - In both trials, the patients had an axillary temperature of 38 degrees Celsius and one moderate or severe respiratory symptom, including cough, nasal congestion, and sore throat. The patients also had systemic symptoms like headache, fever, chills, muscle or joint pain, and fatigue.
Trial Results:
Trial 1:
The patients who received Baloxavir marboxil showed improvements in the influenza symptoms in 63 hours compared to the control group, which received the placebo and showed improvements in 83 hours.
Trial 2:
The adolescent subjects who received Baloxavir marboxil showed alleviation of the symptoms in 54 hours compared to the control group who received the placebo and showed alleviation of the symptoms in 93 hours.
Post-exposure Prophylaxis of Influenza:
Trial:
A phase three randomized double-blind, multicenter, and a placebo-controlled trial was done in 607 patients to evaluate the efficacy of Baloxavir marboxil in the prevention of influenza in subjects who were household contacts of influenza-infected patients. The trial participants received 40 or 80 mg of Baloxavir marboxil according to their weight.
Trial Results:
In patients 12 years of age or above, there was a significant reduction in the proportion of household contacts with laboratory-confirmed clinical influenza from 13 % in the placebo group to 1 % in the group that received Baloxavir marboxil.













