Overview:
Lopinavir and Ritonavir are prescription or fixed-dose combination antiretroviral medications used to treat and prevent HIV in adults and children. Lopinavir is usually combined with a low dose of Ritonavir. It can be used with other antiretroviral drugs to manage HIV infection. In addition to this, Lopinavir and Ritonavir combination therapy can be used to prevent needlestick injuries and other potential exposures.
The drug is taken orally and is available as a tablet, capsule, or oral solution. The most significant advantage of the Lopinavir and Ritonavir combination therapy is that it does not allow the HIV infection to spread from one person to the other. Though Lopinavir and Rotinavir combination therapy does not permanently cure AIDS (acquired immunodeficiency syndrome), it can be effectively used in children and adults diagnosed with HIV to prevent further exposure and transmission.
Drug Development and Approval Timeline:
-
Abbott laboratories initially developed Lopinavir to improve the properties of the previously developed protease inhibitor, Ritonavir.
-
Studies report that Abbott laboratories used the advanced protein source for X-ray crystallography to determine the viral protein structure. These structures helped the researchers know how protease inhibitors can target and inhibit viral replication.
-
The researchers recommended Lopinavir and Ritonavir combination therapy because Lopinavir demonstrated low bioavailability when given alone.
-
Finally, Lopinavir and Ritonavir combination therapy was approved by the United States Food and Drug Administration (FDA) on 15th September 2000.
-
The combination therapy was approved in Europe on 19th March 2001.
Information About Lopinavir and Ritonavir:
How Do Lopinavir and Ritonavir Combination Therapy Work to Treat HIV?
Lopinavir and Ritonavir combination therapy is used against HIV due to its protease inhibition. Most viruses encode proteases as they play a significant role in their life cycle. Therefore, the main mechanism of action of protease inhibitors like Lopinavir and Ritonavir combination therapy is to recognize these proteases.
After recognition, the drug competitively binds to the substrate site of proteases. Hence, the drug is responsible for the post-translational proteolysis of the precursor of polyproteins. In addition, the Lopinavir and Ritonavir combination therapy inhibits functional viral protein production. However, the viral proteins function correctly and undergo replication, maturation, and transcription, the inhibition results in the production of immature viral particles. As a result, the immature viral particles are unable to survive and also lack the potential to trigger an infection.
Safety and Efficacy of Lopinavir and Ritonavir:
Lopinavir and Ritonavir combination therapy belongs to the family of protease inhibitors. Studies show that they are the best drugs against HIV infection. This is because combination therapy blocks the final step of HIV infection.
This implies that the drug inhibits the virion assembly with proven efficacy against HIV. Hence, a combination of Lopinavir and Ritonavir is commonly used as a boosted protease inhibitor for managing HIV infection. However, Lopinavir cannot be administered alone because of its low bioavailability and extensive metabolism by the CYP3A4 isoenzymes. So, the drug has to be coadministered with Ritonavir to achieve the desired results.
What Are Some of the Medical Uses of Lopinavir and Ritonavir Combination Therapy?
Lopinavir and Ritonavir are used along with other medications to control HIV infection. This combination therapy helps decrease the levels of HIV in the body so that the immune system can function correctly. It also lowers the chances of a person acquiring an HIV infection, complications, and other new infections.
Ritonavir has a synergistic effect on Lopinavir, meaning the drug boosts the Lopinavir levels and allows it to function better. An important point to be noted is that Lopinavir and Ritonavir do not cure AIDS but reduce the risk of disease spread. Hence, the patient must follow all the preventive measures and take the appropriate medications to cure HIV.
Dosage and Administration:
Lopinavir and Ritonavir tablets can be taken with or without food. The patient must swallow the whole tablet without crushing, chewing, or breaking it. However, Lopinavir and Ritonavir oral solutions must be taken with food.
Adult Patients:
-
Lopinavir and Ritonavir tablets 400/100 mg (administered as two 200/25 mg tablets) are to be taken twice daily.
-
Lopinavir and Ritonavir oral solution 400/100 mg (5 mL) is to be taken twice daily.
-
Lopinavir and Ritonavir tablets 800/200 mg must be given once daily in patients exhibiting less than three Lopinavir-resistance-associated substitutions.
-
Lopinavir oral solution 800/200 mg (10 mL) is given once daily in patients exhibiting less than three Lopinavir-resistance-associated substitutions.
Note: Lopinavir and Ritonavir tablets and oral solution should not be administered as a once-daily regimen, along with other drugs like Efavirenz, Nelfinavir, and Nevirapine.
-
An increase in the dosage is recommended for all the patients who use Lopinavir and Ritonavir tablets. The recommended dosage of this combination therapy is 500 and 125 mg twice daily in combination with Efavirenz, Nelfinavir, and Nevirapine.
-
The rule mentioned above applies to the Lopinavir and Ritonavir oral solution. So, the recommended dosage of this combination therapy is 533 and 133 mg (6.5 mL) twice daily in combination with Efavirenz, Nelfinavir, and Nevirapine.
Tablets for Pediatric Population:
Lopinavir and Ritonavir tablets must be administered once daily in children under 18. The oral solution should be avoided in neonates before they reach the postmenstrual age of 42 weeks and the postnatal age of 14 days. The doctor must pay special attention to the dosage of an oral solution of Lopinavir and Ritonavir.
In addition, one must be careful about the transcription of the medication order, dispensing information, and dosing instructions to minimize the risk of a drug overdose. The doctor must remain careful while administering the drug to infants and children from 14 days to six months old. The total amount of propylene glycol and alcohol to be administered to these patients must be considered. The doctor must calculate the dosage of Lopinavir and Ritonavir based on the child's body weight or surface area (BSA).
The body surface area can be calculated as follows:
Body Surface Area (Meter Square) =√Height (in centimeters) x weight (in Kg)/ 3600.
The Lopinavir and Ritonavir dose can be calculated based on the body surface area or weight as follows:
Based on the Patient's Weight:
-
Lopinavir Dosage (mg) = Patient Weight (m2) × Prescribed Lopinavir dose (mg/m2).
Based on the Patient's Body Surface Area:
-
Lopinavir Dose (mg) = Patient BSA (m2) × Prescribed Lopinavir dose (mg/m2).
If Lopinavir and Ritonavir oral solutions are to be administered, the volume can be calculated as follows:
-
Volume of Lopinavir and Ritonavir Solution (mL) = Administered Lopinavir dose (mg)/80 (mg/mL).
The oral solution must be administered only using a calibrated dosing syringe. The doctor must assess the child's ability to swallow the tablets before administering them. Lopinavir and Ritonavir oral solution must be administered if the child cannot swallow the tablets.
14 Days to Six Months:
The recommended dosage of Lopinavir and Ritonavir oral solution for children 14 to six months old is 16/4 mg/kg or 300/75 mg/m2 twice daily. However, this combination therapy must not be administered to children below six months along with other drugs like Efavirenz, Nevirapine, or Nelfinavir.
For Children Six Months to 18 Years of Age:
Oral Solution Dosage:
The recommended dosage of the oral solution of Lopinavir and Ritonavir is 230/57.5 mg per m2 twice daily. However, it must not exceed the recommended adult dosage of 400/100 mg twice daily. Based on the weight, the recommended oral solution dosage for patients weighing below 15 kg is 12/3 mg per kg twice daily. In contrast, the dosage for patients above 15 kg to 40 kg is 10/2.5 mg per kg twice daily.
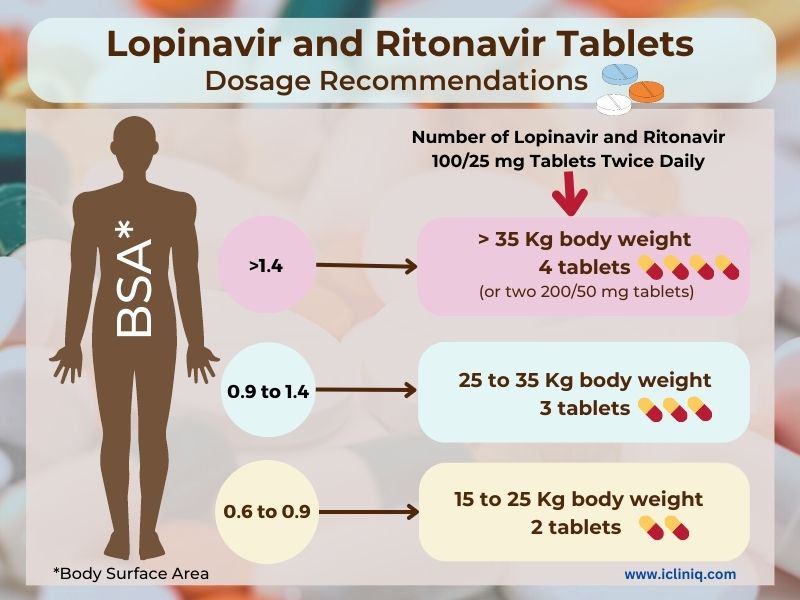
Dosage Recommendations for Lopinavir and Ritonavir Tablets:
The table below describes the dosage recommendations for children six months to 18 years of age based on their body surface area or body weight:
Information About HIV:
HIV is an acronym for human immunodeficiency virus, which attacks the human body's immune system. If left untreated for prolonged periods, it can cause AIDS. The infection mainly spreads through unprotected intercourse, contaminated blood transfusion, and needlestick injuries.
Unfortunately, the infected person cannot completely get rid of HIV as there is no effective and permanent cure for this condition. However, antiretroviral medications can be taken to reduce the viral load from the body and slow down the disease progression. In addition, the patients can take antiretroviral medications to increase their life span and prevent the transmission of viral particles to their partners.
Signs and Symptoms of HIV:
Some patients develop a flu-like illness within two to four weeks of acquiring the virus. This illness is known as the primary or acute HIV infection. Which may last for a few weeks.
The signs and symptoms of HIV are listed below:
-
Headache.
-
Muscle and joint pain.
-
Sore throat.
-
Painful sores in the mouth.
-
Night sweats.
-
Weight loss.
For Patients:
What Is Lopinavir and Ritonavir Combination Therapy?
Lopinavir and Ritonavir combination therapy is a prescription medication used to treat HIV-1 infection in adults and children 14 days of age and above. AIDS occurs when HIV progresses to the advanced stages. Lopinavir is used along with Ritonavir to reduce the amount of virus in the bloodstream. In addition, the drug helps elevate the white blood cells, also known as CD4T cells.
These cells are an important body defense system as they fight off infectious agents. Thus, reducing the HIV viral load and elevation in the CD4 count improves the working of the immune system. It also reduces the risk of other opportunistic infections and death when the body has a weak immune system. Nothing has been known about the drug's efficacy in children below 14. However, the patient must remain careful as Lopinavir and Ritonavir combination therapy do not cure the HIV infection. So, the risk of acquiring other infections like pneumonia and herpes persists.
What Should the Patient Inform the Doctor Before Taking Lopinavir and Ritonavir Combination Therapy?
Before taking the combination therapy, the patient must inform the doctor if he or she has the following:
-
Heart problems, including congenital long QT prolongation syndrome.
-
Pancreatic diseases.
-
Liver diseases, including hepatitis B or C.
-
Diabetes.
-
Hemophilia. The patients taking Lopinavir and Ritonavir combination therapy might have increased bleeding.
-
Low potassium levels.
-
Conceived or planning to become pregnant in the long run. However, no information is available on the effects of Lopinavir and Ritonavir on the unborn baby.
-
Taking or planning to take prescription, over-the-counter drugs, vitamins, and herbal supplements.
There is a risk of drug interaction, so the patient must be careful and inform the doctor if he takes the following:
-
Medications to manage HIV.
-
Estrogen-based birth control pills. Lopinavir and Ritonavir reduce the efficacy of estrogen-based contraceptive pills, so the patient must use an alternative form of birth control.
-
Medications that prevent organ transplant rejection.
-
Anticancer drugs.
-
Amiodarone.
-
Sildenafil.
-
Atorvastatin.
-
Avanafil.
-
Tadalafil.
-
Bepridil.
-
Budesonide.
-
Carbamazepine.
-
Clarithromycin.
-
Fentanyl.
-
Disulfiram.
-
Dexamethasone.
-
Itraconazole.
-
Fluticasone.
-
Lidocaine.
-
Metronidazole.
-
Quinidine.
-
Salmeterol.
-
Trazodone.
-
Valproate.
-
Warfarin.
The patient must keep a list of all his medications and submit the same to the doctor to avoid complications.
For Patients Planning to Become Pregnant:
The patients planning to conceive and take antiretroviral medications must participate in the pregnancy registry. This registry aims to gather information about the baby's and the patient's health.
For Lactating Females:
It is unknown whether the drug can pass from the mother's milk to the baby. However, HIV-1-infected mothers must avoid breastfeeding to prevent the transmission of infection.
How Should the Patient Take Lopinavir and Ritonavir Combination Therapy?
-
The patient must take combination therapy daily as prescribed by his doctor.
-
Set up a dosing schedule and follow it regularly.
-
Do not make changes to the treatment schedule without consulting the doctor.
-
Swallow the Lopinavir and Ritonavir tablets on the whole without crushing, breaking, or chewing them.
If the patient is taking Didanosine Lopinavir and Ritonavir simultaneously:
-
Didanosine can be taken with the Lopinavir and Ritonavir tablets without food.
-
Didanosine must be taken one or two hours later if the patient takes Lopinavir and Ritonavir oral solution.
-
Do not miss the drug dose, making the virus difficult to treat. The patient can take the missed dose immediately if he forgets. Follow the schedule properly to avoid any confusion regarding the drug dose.
-
The patient must visit the emergency room if he takes an extra drug dose.
-
If the child has been recommended to take Lopinavir and Ritonavir, inform the doctor about his weight changes.
-
The oral solution of the drug contains alcohol and propylene glycol in large amounts, so it should not be given to babies younger than 14 days of age.
-
If the child drinks more than the prescribed amount of the solution, take him to the doctor immediately.
-
Avoid taking Metronidazole or Disulfiram with Lopinavir and Ritonavir, as the patient can experience severe vomiting and nausea if he takes these medications.
What Are Some of the Possible Side Effects of Lopinavir and Ritonavir Combination Therapy?
-
Pancreatitis - It is a condition wherein the pancreas becomes inflamed. People taking Lopinavir and Ritonavir combination therapy might experience this condition frequently, resulting in death. The patient has a higher chance of acquiring pancreatitis if he had it before. The patient must inform the doctor if he has abdominal pain, vomiting, or nausea while taking the drug, as these symptoms are suggestive of pancreatitis.
-
Hepatic Problems - Hepatic or liver problems are commonly encountered in patients taking this combination therapy. The doctor should evaluate the patient for hepatitis B or C and do liver function tests and blood tests before initiating the therapy.
The patient must inform the doctor if he experiences the following symptoms:
-
Loss of appetite.
-
Yellowish discoloration of the skin and the whites of the eyes.
-
Dark urine.
-
Pale-colored stools.
-
Abdominal pain.
-
Itchy skin.
-
Diabetes - Diabetics who take protease inhibitors must remain careful as the drug might worsen their symptoms. The patient must consult the doctor if he has excessive thirst or increased urinary frequency.
-
Immune Reconstitution Syndrome - Changes in the immune system can occur in patients who take HIV medications. The immune system might strengthen and fight off infections.
-
Changes in Body Fat Levels - Some patients might observe increased fat in their neck, upper back, trunk, and breasts. Fat loss from the legs, arms, and face might also occur.
Some of the other common side effects of Lopinavir and Ritonavir combination therapy are listed below:
-
Skin rashes.
-
Increased bleeding time in hemophiliacs.
-
Diarrhea.
-
Nausea.
-
Elevated fat levels in the blood.
-
Vomiting.
Storage of Lopinavir and Ritonavir Tablets:
-
The tablets must be stored at room temperatures between 59 to 86 degrees Fahrenheit.
-
Do not keep the tablets outside the container for more than two weeks, especially in a humid environment. Make sure the lid of the container is tightly closed.
Storage of Lopinavir and Ritonavir Oral Solution:
-
The oral solution must always be stored in a refrigerator between 36 to 46 degrees Fahrenheit. Make sure the oral solution is used only until the expiry date mentioned on the label.
-
The oral solution must be used within two months if stored at room temperature.
-
The drug solution must be kept away from high heat.
-
Discard outdated medications immediately.
General Information Regarding Lopinavir and Ritonavir:
The patient must read the medication guide carefully before taking the drug. This is because the drug might be recommended for purposes other than those mentioned in the patient information leaflet. In addition, the patient must avoid administering the drug to people having similar symptoms because they might experience serious side effects. Hence, read the information leaflet carefully and consult the doctor before taking the drug.
For Doctors:
Description:
Lopinavir and Ritonavir are used in combination to treat HIV infection. The main action of Lopinavir is to inhibit HIV-1 protease. Ritonavir does not allow the CYP3A-induced metabolism of Lopinavir, resulting in increased plasma levels of Lopinavir. The pharmacological properties of the drug are listed below:
Lopinavir:
-
Chemical Formula - [1S-[1R*,(R*), 3R*, 4R*]]-N-[4-[[(2,6- dimethylphenoxy)acetyl]amino]-3-hydroxy-5-phenyl-1-(phenylmethyl)pentyl]tetrahydro-alpha-(1-methylethyl)-2-oxo-1(2H)-pyrimidineacetamide.
-
Molecular Formula - C37H48N4O.
-
Molecular Weight - 628.80.
Ritonavir:
Ritonavir is a white or light tan powder freely soluble in methanol, ethanol, and isopropanol. In addition, the drug is practically insoluble in water.
The pharmacological properties of the drug are listed below:
-
Chemical Formula - 10-hydroxy-2-methyl-5-(1-methylethyl)-1- [2-(1-methylethyl)-4-thiazolyl]-3,6-dioxo-8,11-bis(phenylmethyl)-2,4,7,12-tetraaza tribeca-13-oic acid, 5-thiazolyl methyl ester, [5S-(5R*,8R*,10R*,11R*)].
-
Molecular Formula - C37H48N6O5S2.
-
Molecular Weight - 720.95.
Indications and Usage:
Lopinavir and Ritonavir combination therapy is used with other drugs to treat HIV-1 infection in adults and children.
The patient must consider the following while initiating the therapy:
-
A good treatment response might be observed if the Lopinavir and Ritonavir combination therapy is co-administered with other active agents.
-
Before taking the drug, the doctor must obtain the patient's genotypic and phenotypic history. This is because the baseline Lopinavir resistance-associated substitutions might alter the virologic response.
Contraindications:
-
Lopinavir and Ritonavir combination therapy is particularly contraindicated in patients who previously demonstrated hypersensitivity reactions to any of the drug's ingredients. The hypersensitivity reactions include Stevens-Johnson syndrome, toxic epidermal necrolysis, erythema multiforme, urticaria, and angioedema.
-
Lopinavir and Ritonavir should not be coadministered with drugs dependent on the CYP3A clearance pathway. Stop taking the drug if it produces life-threatening symptoms.
-
The drug should not be used with potent CYP3A inducers that reduce the virologic response and increase the chances of cross-resistance and drug interactions.
Clinical Pharmacology:
Mechanism of Action:
Microbiology:
Lopinavir is a potent inhibitor of HIV-1 protease that blocks the cleavage of Gag-Pol polyprotein, producing non-infectious and immature viral particles.
Antiviral Activity:
The antiviral activities of the drug against the HIV strains were evaluated in acutely infected lymphoblastic cell lines and lymphocytes in the blood. The combination therapy demonstrated antagonistic activity with Nelfinavir and synergistic activity with Amprenavir, Atazanavir, and Indinavir.
Resistance:
Numerous HIV-1 isolates were observed in cell cultures, demonstrating reduced Lopinavir susceptibility. However, the same was not observed with Ritonavir. In addition, resistance to Lopinavir and Ritonavir combination therapy was noted in patients previously treated with other protease inhibitors.
Cross-Resistance Studies:
HIV-1 protease inhibitors have demonstrated varying degrees of cross-resistance. However, not much information is available related to the cross-resistance of the virus and their decreased susceptibility to Lopinavir and Ritonavir.
Pharmacokinetics:
The pharmacokinetic properties of Lopinavir and Ritonavir have been evaluated in healthy adult patients and infected ones. However, no clinically significant differences were noted between the two groups. CYP3A is responsible for the complete metabolism of Lopinavir, whereas Ritonavir inhibits this metabolism, increasing Lopinavir's plasma levels. Studies report that administering Lopinavir and Ritonavir 400/100 mg twice daily yields a mean steady-state plasma concentration 15 to 20 times higher than Ritonavir in patients infected with HIV.
Absorption:
A pharmacodynamic study observed that multiple dosing with Lopinavir and Ritonavir produced a peak plasma concentration of 9.8 +/- 3.7 micrograms per mL. The steady-state concentration of the drug before the morning dose was 7.1 +/- 3.9 micrograms per mL.
Effect of Food on the Oral Absorption of Lopinavir and Ritonavir:
Lopinavir and Ritonavir Tablets:
The tablets can be taken with or without food because the patients did not demonstrate any clinically significant changes related to the maximum concentration of the drug.
Lopinavir and Ritonavir Oral Solution:
The maximum concentration increased by 54 % for patients who took the oral solution under fasting conditions. When the oral solution was taken with a high-fat meal, the maximum concentration of Lopinavir increased by 56 %. Hence, the oral solution must be taken with food to enhance bioavailability and minimize the risk of pharmacokinetic variations.
Distribution:
Lopinavir is 98 to 99 % bound to the plasma proteins at a steady state. It binds to albumin and alpha-1acid glycoprotein (AAG). However, the drug has a higher affinity for AAG. Lopinavir binding concentration remains constant when administered twice daily in healthy volunteers and HIV-1-positive patients.
Metabolism:
In vitro studies with human liver and microsomes indicate that Lopinavir mainly undergoes oxidative metabolism. It is metabolized by the hepatic cytochrome P450 and exclusively by the CYP3A isozymes.
Elimination:
2.5 % of the administered dose of Lopinavir can be observed in the urine and feces after eight days of administering the drug. However, after the drug has been administered multiple times, less than 3 % of it is excreted unchanged in the urine. Hence, the observed oral clearance of Lopinavir is 5.98 +/- 5.75 L/hour.
Effects on the Electrocardiogram:
A randomized and placebo-controlled trial was done to evaluate the effect of Lopinavir and Ritonavir on QTcF interval. The mean time differences in the QTcF intervals between placebo and Lopinavir were 5.3 and 15.2 milliseconds, respectively, compared to Lopinavir and Ritonavir 400/100 mg and 800/200 mg.
Chemical Taxonomy:

Overdosage:
Incidences of overdosage have been reported with Lopinavir and Ritonavir combination therapy. For example, a 2.1 kg infant administered Lopinavir and Ritonavir 6.5 mL suffered from a fatal cardiogenic shock.
In addition, the following events have been observed due to the drug overdose:
-
Complete AV (atrioventricular) block.
-
Lactic acidosis.
-
Cardiomyopathy.
-
Acute kidney failure.
Only supportive measures, including monitoring the patient's vitals and observing the patient's clinical status, are available to manage Lopinavir and Ritonavir overdose. Unfortunately, there is no specific antidote available to treat drug overdose.
Non-clinical Toxicology:
Carcinogenesis, Mutagenesis, and Impairment of Fertility:
Carcinogenesis:
The carcinogenic potential of Lopinavir was evaluated during the animal studies by oral gavage administration for 104 weeks. The results demonstrated an increase in the benign hepatocellular adenomas and carcinomas at doses 1.6 to 2.2 times higher than the human exposure. However, nothing has been known about the carcinogenic potential of Ritonavir during animal studies.
Mutagenesis:
Neither Lopinavir nor Ritonavir demonstrated clastogenic or mutagenic effects during the in vitro and in vivo assays, including the Ames bacterial reverse mutation test, mouse micronucleus test, and chromosomal aberration tests in human lymphocytes.
Impairment of Fertility:
No significant effects on fertility were noted when Lopinavir and Ritonavir were used in the 2:1 ratio during animal studies.
Composition of Lopinavir and Ritonavir Combination Therapy:
Active Ingredients - Ritonavir and Lopinavir.
Inactive Ingredients:
-
Lopinavir 200 mg and Ritonavir 50 mg Tablets: Copovidone, colloidal silicon dioxide, sodium stearyl fumarate, and sorbitan monolaurate. The film coating comprises titanium dioxide, hypromellose, polyethylene glycol 400, polyethylene glycol 3350, polysorbate 80, hydroxypropyl cellulose, colloidal silicon dioxide, yellow ferric oxide 172, and talc.
-
Lopinavir 100 mg and Ritonavir 25 mg Tablets: Sodium stearyl fumarate, sorbitan monolaurate, copovidone, and colloidal silicon dioxide. The film coating comprises titanium dioxide, talc, polyvinyl alcohol, polyethylene glycol 3350, and yellow ferric oxide E172.
-
Lopinavir and Ritonavir Oral Solution: Acesulfame potassium, citric acid, glycerin, high fructose corn syrup, Magnasweet-110 flavor, natural and artificial vanilla flavor, polyoxyl 40 hydrogenated castor oil, artificial cotton candy flavor, povidone, sodium chloride, sodium citrate, water, peppermint oil, alcohol, propylene glycol, saccharin sodium, and menthol.
Dosage Forms and Strength:
-
Lopinavir 200 mg/ Ritonavir 50 mg Tablets - They are yellow, ovoid, and film-coated tablets embossed with "a" logo on one side and code KA on one side.
-
Lopinavir 100 mg/ Ritonavir 25 mg Tablets - They are pale yellow colored ovoid tablets embossed with "a" logo and code KC on one side.
-
Lopinavir and Ritonavir Oral Solution - The solution is light yellow or orange colored and contains 400 mg Lopinavir/ 100 mg Ritonavir per 5 mL.
Warnings and Precautions:
Risk of Toxicity in Preterm Infants
Lopinavir and Ritonavir oral solution contains propylene glycol and alcohol. When the solution is administered with propylene glycol, ethanol inhibits the propylene glycol metabolism resulting in elevated drug concentrations.
Preterm neonates are at a higher risk of propylene-glycol-associated adverse reactions because of their diminished ability to metabolize propylene glycol, leading to its accumulation. Therefore Lopinavir and Ritonavir oral solutions must not be used in preterm neonates as nothing has been known about their safety and efficacy. The doctor must assess the risk-benefit ratio before administering the oral solution in case of emergencies.
Pancreatitis
Pancreatitis has been noted in patients receiving Lopinavir and Ritonavir therapy. These patients also developed elevations in triglyceride levels. Fatalities were reported in certain cases.
Patients with a medical history of HIV-1 infections are at a higher risk of pancreatitis. However, nausea, vomiting, and abdominal pain are not suggestive of pancreatitis. Such patients must be evaluated thoroughly, and the therapy must be suspended.
Hepatotoxicity
Patients suffering from hepatitis B or C and presenting with elevated transaminase levels before the treatment are at a higher risk of developing hepatotoxicity. Hepatic dysfunction and fatalities have been reported in patients with advanced HIV-1 infections after the drug was launched. Some patients presented with elevated bilirubin and transaminase levels seven days after initiating Lipoinavir/ Ritonavir therapy. Therefore blood tests and liver function tests must be done before initiating the combined therapy.
Prolongation of the QT Interval
Patients with congenital long QT syndrome must refrain from using the Lopinavir and Ritonavir therapy, as QT interval prolongation has been reported after the drug was launched in the market.
Hyperglycemia
During the post-marketing surveillance of the drug in HIV-1 infected patients, exacerbations of pre-existing diabetes or hyperglycemia and new-onset diabetes have been reported in HIV-1 infected patients. Such patients might need dose adjustments or the initiation of therapy with oral hypoglycemic agents.
Immune Reconstitution Syndrome
This syndrome has been commonly observed in patients who received combination therapies, including Lopinavir and Ritonavir. The patients whose immune system responds to indolent and other residual opportunistic infections might require further evaluation and treatment.
Elevations in Lipid Levels
Some patients presented with elevated triglycerides and cholesterol levels. Hence, appropriate triglyceride and cholesterol tests must be done before initiating the Lopinavir and Ritonavir therapy in such patients.
Fat Redistribution
The following have been noted related to fat redistribution in patients:
-
Central obesity.
-
Cervical hump or dorsocervical fat enlargement.
-
Peripheral wasting.
-
Cushingoid appearance.
-
Facial wasting.
What Are Some of the Adverse Reactions of Lopinavir and Ritonavir Combination Therapy?
The following adverse reactions to Lopinavir and Ritonavir combination therapy were observed when the drug was launched on the market:
Blood and Lymphatic Disorders:
-
Anemia.
-
Lymphadenopathy.
-
Neutropenia.
-
Leukopenia.
Heart Disorders:
-
Myocardial infarction.
-
Atrioventricular block.
-
Tricuspid valve incompetence.
Ear Disorders:
-
Tinnitus.
-
Vertigo.
Eye Disorders:
-
Visual impairment.
Gastrointestinal Problems:
-
Nausea.
-
Diarrhea.
-
Vomiting
-
Abdominal pain.
-
Colitis.
-
Gastroenteritis.
-
Hemorrhoids.
-
Gastroesophageal reflux disease.
-
Constipation.
-
Flatulence.
-
Gastritis.
-
Duodenitis.
-
Gastrointestinal ulcers.
-
Fecal incontinence.
General Problems:
-
Fatigue.
-
Asthenia.
Hepatobiliary Problems:
-
Hepatomegaly.
-
Hepatitis.
-
Cholangitis.
-
Steatosis.
Immunological Disorders:
-
Urticaria.
-
Angioedema.
-
Immune reconstitution syndrome.
Infestations and Infections:
-
Hypertriglyceridemia.
-
Decreased weight.
-
Reduced appetite.
-
Lactic acidosis.
Musculoskeletal Problems:
-
Myalgia.
-
Back pain.
-
Rhabdomyolysis.
-
Osteonecrosis.
Neurological Disorders:
-
Migraine.
-
Dizziness.
-
Insomnia.
-
Nausea.
-
Dizziness.
-
Tremors.
-
Convulsions.
Psychiatric Disorders:
-
Anxiety.
-
Decreased libido.
Renal Problems:
-
Hematuria.
-
Renal failure.
-
Nephritis.
Dermatologic Disorders:
-
Maculopapular rashes.
-
Lipodystrophy.
-
Eczema.
-
Seborrhic dermatitis.
-
Night sweats.
-
Pruritus.
-
Alopecia.
-
Vasculitis.
Drug Interaction Studies:
The table below describes the established and other potentially significant drug interactions of Lopinavir and Ritonavir:
Concomitant Drug Name
Drug Interactions

Use in Specific Populations:
Pregnancy:
Lopinavir and Ritonavir combination therapy is a pregnancy category C drug, but no well-controlled and adequate studies have been conducted on pregnant females. Therefore, they should only be used in pregnant females after carefully assessing the risk-benefit ratio.
Lactation:
The CDC (Center for Disease Control and Prevention) reports that HIV-1-infected mothers must not breastfeed their babies to avoid the risk of viral transmission. However, no information is available on whether Lopinavir and Ritonavir can pass from the mother's milk to the baby.
Pediatric Population:
The safety and efficacy of the drug have not been known in children below 14 days of age. An open-label multicenter trial was done on 100 patients six months old and above. The trial results revealed that the drug could be safely administered in patients six months to 18 years of age.
Geriatric Population:
The clinical trials did not include a sufficient number of patients above 65. Hence, not much information is available regarding the safety and efficacy of Lopinavir and Ritonavir in such patients. Hence, combination therapy must be administered cautiously in such patients. In addition, these patients must be evaluated for hepatic dysfunction and other side effects.
Hepatic Impairment:
The liver particularly metabolizes Lopinavir and Ritonavir. Hence care must be taken before administering the drug to patients with hepatic dysfunction, as there can be a sudden increase in the blood levels of Lopinavir.
Clinical Trial Studies:
Adults Patients Without Receiving Any Antiretroviral Therapy Before:
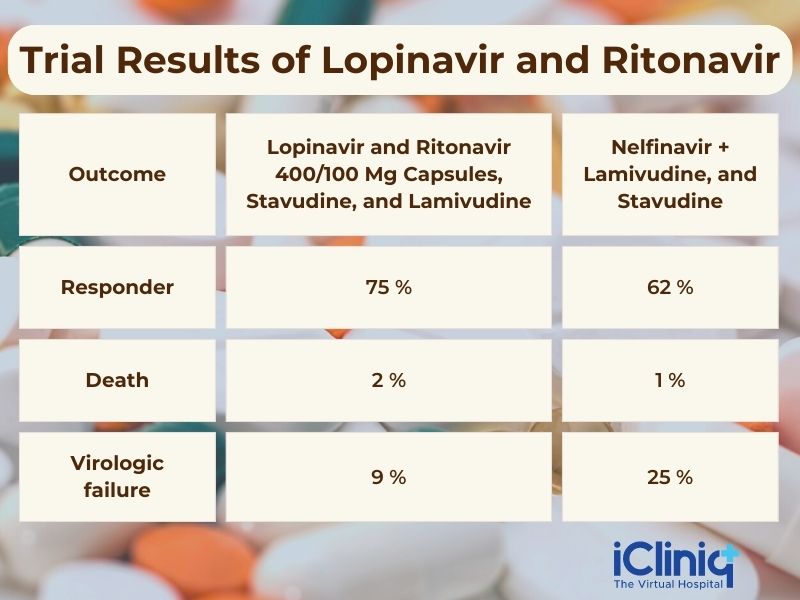
A randomized, double-blind, and a multicenter trial was done to compare the treatment with Lopinavir and Ritonavir 400/100 mg capsules, Stavudine, and Lamivudine versus Nelfinavir thrice daily + Lamivudine, and Stavudine. This trial was done on 653 patients with a mean age of 38.
Trial Results:
The trial results are listed in the table below:
Outcome.
Lopinavir and Ritonavir 400/100 MG Capsules, Stavudine, and Lamivudine.
Nelfinavir + Lamivudine, and Stavudine.
Trial Results:
The trial results are listed in the table below: