Overview: Pimavanserin is a typical antipsychotic used for hallucinations and delusions in Parkinson's disease psychosis (PDP) cases. The FDA (Food and Drug Administration) approved the drug in 2016. This is the first-ever drug the FDA approved for Parkinson's disease psychosis. Due to the actions of Pimavanserin on serotonin receptors and its inability to affect dopamine, it treats the symptoms of hallucinations and delusions without causing extrapyramidal symptoms. The drug is still under approval for treating dementia-related psychosis.
Uses of Pimavanserin:
The drug is used to treat certain mental and mood disorder symptoms related to Parkinson's disease. It helps to reduce the symptoms like hallucinations where the person sees and hears things that are not even there and delusions which are false beliefs. Although Pimavanserin is an antipsychotic drug, it works differently from other drugs of the same class.
Precautions for Pimavanserin:
-
The drug should be avoided in cases of allergies as it may contain some ingredients which may lead to allergy.
-
The drug should be taken wisely in cases of kidney diseases.
-
The drug Pimavanserin can affect the heart rhythm by affecting the QT prolongation. QT prolongation is rarely fatal and may cause increased or irregular heartbeats. The chances of QT prolongation increase if the patient suffers from certain medical conditions or is under some drug that can lead to QT prolongation.
-
Old patients are more susceptible to QT prolongation in cases of Pimavanserin administration.
-
During pregnancy, the drug is recommended only if needed and with proper discussion with the doctor.
What Is Parkinson’s Disease?
It is a progressive nervous disorder that affects the movements of an individual. The disease symptoms generally start with a barely noticeable tremor in the hands. The tremors get more common and may lead to stiffness or slowing of the body's muscles. In the early days of the disease, the face of the patient may show little or no expression. The arms will not swing when the patient walks, and the speech may become slurred and soft. The causes of Parkinson’s disease include the breaking down of certain nerve cells in the brain. Most symptoms are due to the loss of neurons which produces a chemical messenger in the brain called dopamine. When dopamine increases in the brain, it causes abnormal activity and impaired movements.
What Are the Symptoms of Parkinson’s Disease?
The signs and symptoms of Parkinson’s disease include;
-
Tremor: The shaking usually starts in the limbs, hands, and fingers. The patient may rub the thumb and forefinger back and forth, known as a pill-rolling tremor.
-
Rigid Muscles: Patients with Parkinson’s disease may have muscle stiffness which can be painful and can limit the range of motion of body parts.
-
Bradykinesia (Slowed Movements): Gradually, with time, the patients with Parkinson’s disease have slow body movements, making it difficult even to perform small activities. The steps might become shorter while walking and even harder to drag feet.
-
Impaired Posture and Balance: The posture might become stooped, and there is a problem balancing the body.
-
Speech Changes: The patient may speak softly, quickly, or hesitate before talking to others.
-
Difficulty in writing and reading.
The treatment of the disease is to control the symptoms with the help of medications. The medications will help to increase the levels of dopamine to help to substitute it. The medications help to manage the walking, movements, and tremor. The speech-language therapist advises the patient on lifestyle changes, including ongoing aerobic exercises, physical therapy, and speech corrections.
For the patients:
Before Taking Pimavanserin:
Some important points should be considered and brought to the doctor's notice before starting Pimavanserin. Such conditions include;
-
Allergy: Any drug allergy or allergy from any other chemicals should be informed to the doctor as the drug may contain some ingredients which may lead to allergy.
-
Kidney Diseases: Drug metabolism and excretion depend on the kidney functioning, and it gets altered if there are any kidney diseases. So it is important to inform the doctor about any kidney issues.
-
Heart-Related Conditions: Pimavanserin is observed to increase the QT prolongation in some patients, which may not be fatal or serious but should always be kept at the doctor's notice. Patients with heart issues should inform the doctor before starting the drug. Even if the patient has a family history of any heart disease, they should be informed by the doctor. Low levels of magnesium and potassium in the body may also increase the chances of QT prolongation, this risk increases when drugs such as diuretics or water pills are taken.
-
Pregnancy and breastfeeding should also be informed to the patient before starting the drug.
How to Take Pimavanserin?
The drug should be taken orally with or without the food as instructed by the doctor. If there is a problem swallowing the capsule, it can be opened, and all the content should be mixed in a tablespoon full of yogurt, applesauce, pudding, or a liquid nutritional supplement. The mixture should be consumed immediately without chewing it and not be saved for future use.
The drug should be taken regularly as per the doctor’s advice for most of its benefits and at the same time every day. The dosage of the drug depends on the symptoms and other medications used by the patients.
Dosage of Pimavanserin:
The drug should be taken at a maximum of 34 mg. According to the doctor, it should be administered as two 17 mg orally with or without food.
Side Effects of Pimavanserin:
These drugs are always given by the doctor because the doctor knows that the drug's benefits are more than the side effects caused by them. Some side effects caused by Pimavanserin are;
-
Mild Side Effects: The normal or mild side effects caused by Pimavanserin include swelling in the ankles, hands, and feet with confusion and nausea. If any of these side effects last longer, they should be informed to the doctor.
-
Serious Side Effects include fast or irregular heartbeat, severe dizziness, and fainting. The doctor should be informed immediately about any of these symptoms.
-
Allergic reactions due to Pimavanserin are rare but can lead to symptoms such as swelling of the face, rashes, itching, dizziness, and trouble breathing.
What if a Dose of Pimavanserin Is Missed?
If a dose of Pimavanserin is missed and there is still a long time for the next dose (say more than 12 hours), the patient can take the dose, but if the time is almost for the next dose, it is advised to take only the next dose and not two doses at a time.
What if There Is an Overdose of Pimavanserin?
In cases of overdose of the Pimavanserin, the patient may feel exhausted and faint. It is advised to contact the doctor in such cases immediately.
Storage of Pimavanserin:
The drug should be stored at room temperature away from light and moisture. The drug should also be kept away from children and pets. The disposal of expired drugs should be done according to doctor or pharmacists' advice.
For Doctors:
Indication:
Pimavanserin is indicated for treating hallucinations (seeing and hearing things that are not actual) and delusions (feeling and seeing imaginary things) related to Parkinson’s associated psychosis.
Pharmacodynamics:
The unique action of Pimavanserin on serotonin receptors improves the signs and symptoms of hallucinations and delusions associated with Parkinson’s disease. Pimavanserin does not worsen the motor functioning of patients with Parkinson’s disease. In clinical studies, it was reported that about 80.5 % of cases treated with Pimavanserin showed improvement in their symptoms.
Mechanism of Action:
Parkinson’s disease psychosis is an imbalance of serotonin and dopamine due to disruption in the normal balance of serotonergic and dopaminergic receptors and the neurotransmitters in the brain. The mechanism of Pimavanserin for treating hallucinations and delusions is not completely established. It can be possible that Pimavanserin acts by inverse agonist and antagonist activities at serotonin receptor (5-HT2C) with minimal effects on serotonin 5- HT2C receptors. Pimavanserin is an antagonist and reverses agonist of serotonin 5- HT2A receptors with high binding affinity and low binding affinity towards 5- HT2C receptors. Pimavanserin cannot bind to dopaminergic, muscarinic, adrenergic, and histaminergic receptors, which helps to prevent the undesirable effects due to typical antipsychotics.
Absorption of Pimavanserin:
In critical studies, the median Tmax for Pimavanserin was six hours, irrespective of the dose. The bioavailability of an oral tablet and the solution of Pimavanserin is almost the same. The major active circulating N- demethylated metabolite, AC-279, is the one that has a median Tmax of six hours.
The Volume of Distribution:
In clinical studies, after a single dose of 34 mg of Pimavanserin, the apparent volume of distribution was 2173 L.
Protein Binding Affinity:
Pimavanserin has a very high protein binding affinity to the human body; it binds almost 95 % human plasma protein.
Metabolism:
The metabolism of Pimavanserin is generally by CYP3A4 and CYP3A5 hepatic cytochrome enzymes and to some extent by CYP2J2, CYP2D6, and some other cytochromes and flavin-containing monooxygenase enzymes. CYP3A4 is known to metabolize Pimavanserin into its active major metabolite AC- 279.
Elimination:
The drug Pimavanserin is eliminated both in the urine and the feces. About 0.54 % of the 34 mg of the drug is excreted unchanged from the urine, and the feces eliminates approximately 1.53 % in about ten days. In clinical studies, less than one percent of the dose was eliminated and its metabolites were recovered in the urine.
Half-Life of Pimavanserin:
The average plasma half-life for Pimavanserin is 57 hours, and that of its active metabolite AC- 279 is 200 hours.
Toxicity:
Pimavanserin is not known to cause serious toxicity in overdose cases. The mild symptoms may include nausea and vomiting. However, cardiovascular monitoring should be started immediately after the overdose with continuous ECG monitoring of the patient. There are no antidotes present for the overdose of Pimavanserin. In cases of overdose, the plasma half-life of 57 hours of Pimavanserin should be considered with a possibility of multiple drug involvement.
Drug Interactions of Pimavanserin:
Some commonly used drugs that can interact with Pimavanserin are:
-
Acetaminophen: The metabolism of Pimavanserin is noticed to increase when it is mixed with Acetaminophen.
-
Albendazole: The metabolism of Pimavanserin decreases when it is mixed with Albendazole.
-
Azithromycin: Pimavanserin's metabolism decreases when combined with Azithromycin.
-
Cyclobenzaprine: The drug is known to increase the neurotoxic activities of Pimavanserin.
-
Dronedarone: The metabolism of Pimavanserin can be increased when mixed with Dronedarone.
-
Fluconazole: The metabolism of Pimavanserin increases in combination with Fluconazole.
Food Interaction of Dupilumab:
- Grapefruit Products: These act as CYP3A4 inhibitors, and thus, the dose of Pimavanserin has to be adjusted if grapefruit or its products are consumed.
- St. John’s Wort: It can induce the CYP3A4 metabolism of Pimavanserin, which further reduces the drug's efficacy.
- The drug can be consumed with or without food. There is no specific condition for that until instructed by the doctor.
Clinical Trials of Pimavanserin:
-
Study for approval of Pimavanserin in treatment of hallucination and delusion due to Parkinson’s disease: the study was demonstrated for six weeks, a randomized, placebo-controlled, and parallel-group phase III study done by Cummings et al.
-
The study randomized 199 patients with an average age of 72 years suffering from Parkinson’s associated psychosis. They received either Pimavanserin 34 mg or a placebo daily. The study's outcome was assessed by a Parkinson’s disease adapted scale to evaluate positive symptoms (SAPS-PD). The group of patients who received Pimavanserin showed significantly improved SAPS-PD score in the sixth week compared to the group which received a placebo.
Relapse prevention study for Pimavanserin in dementia associated psychosis:
-
Purpose: The motive behind the surgery is the evaluation of the efficacy of the drug Pimavanserin compared to the placebo for preventing the relapse of the psychotic symptoms in subjects with dementia-related psychosis in cases who responded to 12 weeks of open-label Pimavanserin.
-
Number of Participants: 392.
- Allocation: Randomization.
Primary Purpose of the Study: Treatment of the cases.

A study about the safety and efficacy of Pimavanserin in patients with Alzheimer’s disease psychosis:
Purpose of Study: To see the efficacy of Pimavanserin in patients who have psychosis related to Alzheimer’s.
The Number of Participants: 181.
Allocation: Randomized.
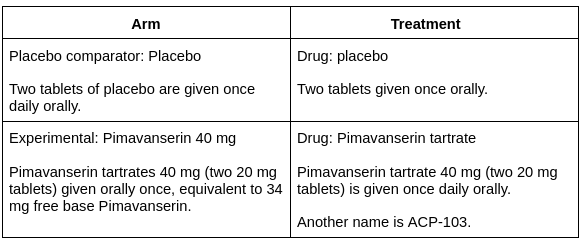
Results: There was an improvement in the symptoms of the patients.
Pimavanserin and Pregnancy:
The drug is generally advised to be avoided in pregnancy until it is imperative to take it. Studies have not proposed any adverse effect on the fetus or mother from the drug Pimavanserin yet.
Pimavanserin and Breastfeeding:
There are no established studies about the passing of the drug into breast milk. However, it is recommended to take the drug only on strict advice and instructions from the doctor.
Pimavanserin in Geriatric Patients:
Pimavanserin in geriatric patients with Parkinson’s increases hospitalization and mortality risk. Thus the drug should be taken only if it is essential and under proper guidance.













