Overview:
Tafasitamab-cxix is a drug used with Lenalidomide to treat refractory or relapsed diffuse B- cell lymphoma in adults (DLBCL). It is a modified CD-19-directed monoclonal antibody that binds to the receptors, including diffuse large B-cell lymphoma, and facilitates cell lysis mediated by apoptosis and immune effector mechanism. It is available as an injection (administered by a healthcare provider only). Tafasitamab received its Food and Drug Administration (FDA) approval on July 31, 2020. Studies assessing the efficacy of the therapy in a multi-centered, open-label, single-arm trial for 12 cycles with the monotherapy indicated that 37 % of the patients showed complete response, whereas 18% of them showed partial response with a confirmed diagnosis (55 %) with DLBCL.
How Does Tafasitamab-cxix Work?
Tafasitamab-cxix binds to the CD19 antigen expressed on the surface of pre-B and mature B cells and several B-cell malignancies, including diffuse large B-cell lymphoma (DLBCL).
Tafasitamab-cxix mediates B-cell lysis after binding to CD19 by inducing apoptosis and immunological effector mechanisms, such as antibody-dependent lymphocytic infiltration (ADLI), antibody-dependent cellular cytotoxicity (ADCC), and the phagocytosis of cells.
Uses:
Tafasitamab-cxix is combined with Lenalidomide to treat diffuse large B-cell lymphoma, relapsed or refractory lymphoma, including disease arising from low-grade lymphoma, and not eligible for autologous stem cell transplant.
What Is the Dosage of Tafasitamab-cxix?
-
Tafasitamab-cxix is administered as an infusion intravenously, and the recommended dose is 12 mg/ kg of body weight. The doctor or nurse will help Tafasitamab-cxix on each cycle of drug therapy as per the standard dosing guidelines.
-
It is given on days 1, 4, 8, 15, and 22 of cycle 1, on days 1, 8, 15, and 22 of cycles 2 and 3, and on days 1 and 15 of cycles 4 to 12.
-
The drug is administered for up to 12 cycles, each lasting 28 days.
-
It is crucial to let the doctor know how well the drug works and if any side effects are experienced.
-
Some side effects may be experienced while on the therapy. The oncologist can prescribe other drugs to treat or prevent adverse reactions to Tafasitamab-cxix.
-
While receiving the infusion, a doctor or nurse will keep a close eye to ensure no negative responses to the medication are experienced.
Warning:
-
Infections- Infections, including opportunistic infections, are likely during and after chemotherapy with Tafasitamab-cxix patients. 81 patients diagnosed with DLCBL indicated pneumonia, nasopharyngitis, bronchitis, urinary tract infections, and respiratory tract infections. Pneumonia (grade 3 or higher) was the most prevalent infection. Therefore, symptoms of the infection should be identified and treated promptly.
-
Infusion- Related Reactions- Tafasitamab-cxix is an infusion. It may cause infusion-related reactions like fever, urticaria, pruritus, rigor, etc. If these symptoms are identified, necessary medical intervention is required.
-
Toxicity to the Fetus- Tafasitamab-cxix has shown to potentially deplete the fetal B-cell population when administered to pregnant women. Therefore, it is crucial to inform women with reproductive ability about the potential risk to the fetus. The use of reliable contraception both while taking Tafasitamab-cxix and for at least three months after the last dosage is advised.
For Patients:
What Is Diffuse Large B-Cell Lymphoma?
Lymphoma is a cancer of the lymphatic system, part of the immune system. It includes the lymph nodes, thymus, spleen, and bone marrow. These organs produce, store, and carry lymphocytes. Lymphocytes are a type of white blood cell that helps the body fight infection.
DLBCL is a cancer that starts in B-lymphocytes. They are made in the bone marrow and mature in the lymph nodes. Diffuse large B-cell lymphoma can occur at any age but is most common in people over 60.
There are two types of DLBCL:
-
High-Grade - This type of DLBCL is more aggressive and proliferates. It is the most common type of DLBCL.
-
Low-Grade - This type of DLBCL grows slowly and is less likely to spread to other body parts.
What Are the Symptoms of Large B-Cell Lymphoma?
The symptoms of DLBCL include
-
Swollen lymph nodes.
-
Weight loss.
-
Tiredness.
-
Loss of appetite.
-
Night sweats.
If any of these symptoms are experienced, consult the doctor. DLBCL is diagnosed with a physical exam, blood tests, imaging tests, and a biopsy.
Learn More About Tafasitamab-cxix:
Before Starting Tafasitamab-cxix:
Given below are a few things to know before starting with Tafasitamab-cxix.
When and Why to Take Tafasitamab-cxix?
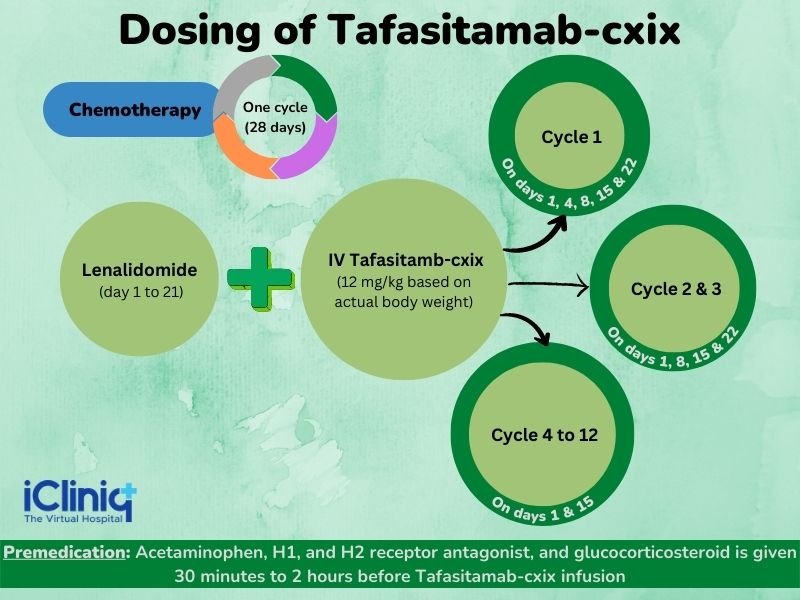
One cycle of chemotherapy corresponds to a period of 28 days. It is to be noted that Tafasitamab-cxix is given along with the drug Lenalidomide.
The chemotherapy regimen with Tafasitamab-cxix is as follows:
-
Cycle 1 - Tafasitamab-cxix on five days (1, 4, 8, 15, and 22) during this cycle.
-
Cycles 2 and 3 - Tafasitamab-cxix is given on four days (1, 8, 15, and 22) within each of these cycles.
-
Cycles 4 to 12 - Tafasitamab-cxix is administered once every two weeks.
-
After 12 Cycles - Tafasitamab-cxix is administered as maintenance therapy.
What Are the Warnings and Precautions of Tafasitamab-cxix?
Inform the doctor of the following medical conditions (if any) before starting with Tafasitamab-cxix:
-
A history of hypersensitivity or allergies to any drugs.
-
Have a current or recent infection.
-
Is expecting a child or intends to get pregnant. Tafasitamab-cxix could be harmful to the unborn child. If pregnant, avoid taking Tafasitamab-cxix therapy because this medication can result in birth abnormalities or even the unborn child's death.
-
If you become pregnant while taking Tafasitamab-cxix, call the doctor right away.
-
Is currently breastfeeding. It is unknown if Tafasitamab-cxix can enter the breastmilk. Avoid breastfeeding while receiving therapy and for at least three months following the last drug dose.
Starting Tafasitamab-cxix:
How to Take Tafasitamab-cxix?
A healthcare professional administers Tafasitamab-cxix in a clinical setting. The doctor will schedule the cycles of chemotherapy. It is essential to meet the doctor regularly for therapy. Also, inform the oncologist regarding any other medication being taken before starting the chemotherapy regimen.
How Long Does the Infusion Take?
The first infusion after premedication will be roughly after 1.5 to 2.5 hours. If the medical team needs to make adjustments while you are receiving treatment, the infusion time may change. Following that, the infusion will take between 90 minutes and two hours.
Things to Do After Taking Tafasitamab-cxix:
Once the chemotherapy begins, always check for the appearance of symptoms of infection, including fever, chills, flushing, headache, difficulty breathing, etc. Report to the doctor right away if any of these symptoms are experienced.
What Are the Side Effects of Tafasitamab-cxix?
The list of side effects of Tafasitamab-cxix is as follows:
Common side effects:
-
Nausea.
-
Vomiting.
-
Diarrhea.
-
Decreased blood count.
-
Fatigue.
-
Cough.
-
Back pain.
-
Muscle spasm.
-
Decreased appetite.
Report to the doctor if these effects worsen or persist for more extended periods.
The severe side effects include
-
Respiratory tract infection.
-
Urinary tract infection.
-
Decreased potassium levels.
-
Unexplained weight loss.
-
Unusual bruising or bleeding.
-
Hypersensitivity reactions - symptoms include rashes, hives, itching, and shortness of breath.
This list does not provide the full side effects. If those mentioned above or negative symptoms appear, report to the doctor immediately.
Dietary Alterations:
There are no specific nutritional alterations for Tafasitamab-cxix. Follow the regular diet unless otherwise specified by the nutritionist or oncologist. Ensure to eat every day and balanced meals, aiding the proper functioning of the body.
What Should Be Done if a Dose Is Missed?
If an appointment for chemotherapy is missed, immediately report it to the clinic. Then, the doctor may reschedule the appointment at the earliest.
What Should Be Done to Treat Tafasitamab-cxix Overdose?
Since Tafasitamab-cxix is delivered in a clinical setting, there are few chances of overdosing. However, if you develop side effects like hives, itching of the lip or tongue, or facial swelling during or after receiving Tafasitamab-cxix infusion, contact the doctor right away.
How to Store Tafasitamab-cxix?
A medical professional is in charge of maintaining the storage of Tafasitamab-cxix, which is delivered at a hospital or clinic.
The storage specifications are as follows:
-
Tafasitamab-cxix is a lyophilized powder for reconstitution.
-
It is sterile, free of preservatives, and available in 200 mg single-dose vials, each packed explicitly in a carton.
-
Store the drug in a refrigerator at 2 to 8 degrees Celsius inside the carton to protect it from direct light.
-
Avoid freezing or shaking the vial.
For Doctors:
Indication:
Tafasitamab-cxix is used to treat diffuse large B-cell lymphoma, relapsed or refractory, including disease arising from low-grade lymphoma, and not eligible for autologous stem cell transplant, in combination with Lenalidomide.
Pharmacology:
The pharmacology of the drug is as follows:
Mechanism of Action:
Tafasitamab-cxix is a monoclonal antibody that binds to and inhibits the activity of CD19, which results in the lysis of B-cells. The pre-B and mature B-lymphocytes express the CD19 surface antigen, which is thought to be essential for both the survival and enhancement of B-cell receptor signaling. Additionally, certain B-cell cancers, including diffuse large B-cell lymphoma (DLBCL), small lymphocytic lymphoma (SLL), and chronic lymphocytic leukemia (CLL), substantially express these surface proteins (DLBCL). Therefore, the drug acts by inducing direct apoptosis, and immune-mediated effector mechanisms, such as antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis, are involved in this process (ADCP).
Dosing:
The general dosing information of Tafasitamab-cxix is as follows:
Premedication: May include Acetaminophen, H1 receptor antagonist, H2 receptor antagonist, and glucocorticosteroid given 30 minutes to 2 hours before Tafasitamab-cxix infusion; for patients not experiencing infusion-related reactions during the first three infusions, premedication is optional for subsequent infusions; if a patient experiences an infusion-related reaction, administer premedications before each subsequent infusion.
-
Severe Renal Impairment (CrCl less than 30 mL/min)- No specific recommendations are available.
-
Mild Hepatic Impairment- There were no discernible variations in the pharmacokinetics of Tafasitamab-cxix that were clinically significant.
-
Moderate to Severe Hepatic Impairment- No specific recommendations are available.
-
Geriatrics - More serious adverse reactions (57%) were reported compared to younger patients (39 %) in clinical studies due to inadequate numbers of patients 65 years or older to determine whether effectiveness differs from younger patients.
-
Myelosuppression - For platelet counts less than 50,000/mcL, withhold Tafasitamab-cxix and Lenalidomide; continue Tafasitamab-cxix at the same dose and Lenalidomide at a lower dose until the platelet count reaches 50,000/mcL or above; monitor CBC every week.
Infusion-Related Reactions:
-
Grade 2 - For moderate infusion-related reactions, restart infusion at no more than 50 % of the reaction's rate once it has subsided or been reduced to Grade 1. Suppose the patient does not experience another reaction within an hour, and their vital signs are stable. In that case, the infusion rate may be increased every 30 minutes as tolerated to the rate at which the reaction occurred.
-
Grade 3 - For severe reactions, restart infusion at no more than 25 % of the rate at which the reaction occurred once signs and symptoms have subsided or have been reduced to Grade 1. Suppose the patient does not experience another reaction within an hour, and their vital signs are stable. In that case, the infusion rate may be increased every 30 minutes as tolerated up to a maximum of 50 % of the rate at which the reaction occurred.
-
Grade 4 - For life-threatening infusion-related reactions, Infusion-related reactions, stop the infusion right away, commence the appropriate medical management, and stop the therapy completely.
Pharmacodynamics:
After eight days of treatment, Tafasitamab-cxix decreased peripheral blood B cell counts by 97 % in individuals with relapsed or resistant DLBCL. Within 16 weeks of treatment, a decrease of 100 % was reached.
Pharmacokinetics:
Observations from the studies indicate the overall maximum serum concentration of Tafasitamab-cxix is 483 μg/mL.
Distribution:
The total volume of distribution of the drug is 9.3 L.
Elimination:
Tafasitamab-cxix had a terminal elimination half-life of 17 days and a clearance of 0.41 L/day.
Specific Populations:
The pharmacokinetics of Tafasitamab-cxix are significantly influenced by body weight (40 to 163 kg), with increased clearance and volume of distribution anticipated with increasing body weight. Age (16 to 90 years), sex, mild to moderate renal impairment (CLcr 30-89 mL/min, and mild hepatic impairment (total bilirubin ULN and AST > ULN, or total bilirubin 1 to 1.5 times ULN and any AST) were not associated with clinically significant differences in the pharmacokinetics of Tafasitamab-cxix It is uncertain how race or ethnicity, severe hepatic impairment (total bilirubin > 1.5 times ULN and any AST), and end-stage renal disease (CLcr 30 mL/min) affect the pharmacokinetics of the drug.
Active Ingredient:
The active ingredient in the infusion is Tafasitamab-cxix.
Inactive Ingredients:
The inactive ingredients include
-
Sodium citrate dihydrate.
-
Trehalose dihydrate.
-
Citric acid monohydrate.
-
Polysorbate 20.
Toxicity:
Tafasitamab-cxix has not been studied for carcinogenicity, mutagenicity, or fertility studies. Animal study data indicate no adverse effects on the male and female reproductive organs in the 13-week repeat-dose general toxicity investigation in cynomolgus monkeys up to the highest dose tested, 100 mg/kg/week.
Warning and Precaution:
-
Hematologic Effects - Tafasitamab-cxix is associated with profound or severe myelosuppression, including anemia, neutropenia, and thrombocytopenia. Consider granulocyte colony-stimulating factor if it occurs; monitoring is recommended, and dose interruption may be necessary.
-
Immunologic Reactions - Infusion-related reactions (e.g., chills, flushing, dyspnea, and hypertension) may occur. Therefore, premedication and monitoring are recommended, and dose interruption, discontinuation, and treatment may be necessary. Also, fatal and severe infections, including opportunistic infections, have been reported during use and following the last dose; monitoring and treatment may be required.
-
Reproductive - This may cause fetal harm due to fetal B cell depletion. Advise pregnant women of the potential risk to the fetus; women of reproductive potential should use effective contraception during treatment and for at least three months after the last dose.
Dosage and Forms:
-
Tafasitamab-cxix is available only as 200 mg intravenous powder for solution.
-
It is a preservative-free, sterile, and slightly yellowish lyophilized powder.
Preparation and Administration of the Drug:
The directions for the reconstitution, dilution and administration are as follows:
Reconstitution:
-
Calculate the dosage (in mg) and the required number of vials.
-
To achieve a final concentration of 40 mg/mL Tafasitamab-cxix, reconstitute each 200 mg Tafasitamab-cxix vial with 5 mL Sterile Water for Injection, USP with the stream directed toward the wall of each vial.
-
Gently stir the vial(s) until they have dissolved completely. Avoid excessive shaking or swirling for up to five minutes before complete breakdown.
-
Check the reconstituted solution visually for any particles or discoloration. The reconstituted solution should have a hue ranging from colorless to faintly yellow. If the solution is hazy, discolored, or contains visible particles, throw away the vial(s).
-
Use the drug solution as soon as it has been reconstituted. If necessary, keep the reconstituted solution in the vial at room temperature ( 20°C to 25°C) or in the refrigerator (2°C to 8°C) for a maximum of 12 hours before dilution. Keep away from light while storing.
Dilution:
-
Based on the desired dose, determine how much (in mL) of the reconstituted 40 mg/mL of the drug solution is needed.
-
From a 250 mL 0.9 % Sodium Chloride Injection USP infusion bag, take out and discard the necessary amount of solution.
-
Withdraw the required drug quantity and gradually dilate the 0.9 % Sodium Chloride Injection USP in the infusion bag to a final concentration of 2 mg/mL to 8 mg/mL.
-
Any unused drug still in the vial should be thrown away.
-
Inverting the IV bag slowly can help it mingle gently. Do not tremble. Before administration, visually check the infusion bag for debris and discoloration with the diluted infusion solution.
-
If the diluted infusion solution is not used right away, it should be kept chilled for up to 18 hours at two °C to 8°C or at room temperature for up to 12 hours at 20°C to 25°C. The period for infusion is included in the storage at room temperature. Keep away from light while storing. Avoid freezing or shaking the reconstituted or diluted infusion solutions.
Administration:
-
Administer the first infusion at a rate of 70 mL/hr for the first 30 minutes, then increase the rate so that the infusion is administered within 1.5 to 2.5 hours; administer all subsequent infusions within 1.5 to 2 hours.
-
No incompatibilities have been observed with polyurethane (PUR) or PVC (polyvinyl chloride) infusion sets.
-
Do not co-administer other drugs through the same infusion line.
Contraindications:
Tafasitamab-cxix is contraindicated in patients with
-
Known history of hypersensitivity to drug components.
-
Pregnant women due to potential harm to the fetus.
Clinical Studies for Tafasitamab-cxix:
-
In an open-label, single-arm, multicenter study, the effectiveness of Tafasitamab-cxix.
-
In conjunction, Lenalidomide was assessed before Tafasitamab-cxix monotherapy. Lenalidomide (25 mg orally on days 1to21 of each 28-day cycle) and Tafasitamab-cxix (12 mg/kg intravenously) were given to patients for a maximum of 12 cycles.
-
The median age of the 71 patients with DLBCL who received the combined therapy was 71 years (ranging from 41 to 86 years); 55% were men, and 100 % had previously undergone a CD20-containing therapy.
-
92 % of patients had their race recorded; 95 % were White, and 3 % were Asian.
-
The median number of past therapies was two, with 51 % having two to four prior lines of treatment, compared to 49% who had only one.
-
Thirty-two patients (45%) were resistant to their most recent therapy and 30 (42%) to Rituximab. Nine patients (13%) had previously had ASCT (autologous stem-cell transplant). Age (47%), refractoriness to salvage chemotherapy (27%), comorbidities (13%), and rejection of high-dose chemotherapy/ASCT were the main reasons patients were not suitable for ASCT.
Results- The efficacy was established as response rate percentage. 37 % of the enrolled patients showed complete response, and 18% with partial response rate.
Drug Interactions:
1. Drug-Drug interactions -
-
Adalimumab.
-
Anakinra.
-
Azathioprine.
-
Chloramphenicol.
-
Filgrastim.
-
Infliximab.
-
Teriflunomide.
-
Topotecan.
-
Ustekinumab.
-
Natalizumab.
-
Tetanus toxoid.
-
Zidovudine.
This list does not include all the drug interactions of Tafasitamab. Check with the pharmacist to know more.
2. Drug- Alcohol Interactions - No data exist on the interaction of alcohol with the drug.
3. Drug-Food Interactions - No serious food interactions are reported.
Other Specifications:
Tafasitamab-cxix in Pregnant and Lactating Women -
Tafasitamab is contraindicated in pregnancy. Women of reproductive potential should be advised to use contraception at least three months after therapy.
There is no information on Tafasitamab-presence in human milk and its effects on nursing infants. Women are advised not to breastfeed while undergoing treatment due to the possibility of significant adverse responses in the breastfed baby.
Tafasitamab-cxix in Pediatrics -
The safety and efficacy of the drug are not yet established in children.
Tafasitamab-cxix in Geriatrics -
Geriatrics were reported to have more serious adverse events than younger patients. However, this is not supported by sufficient data due to the limited number of patients above 65 years enrolled in the study.












