Overview:
Darifenacin is a medication used to treat incontinence caused by an overactive bladder. It is available as an extended-release oral tablet. Darifenacin is a selective muscarinic (M3 receptor) antagonist that relaxes the bladder's smooth muscles, thereby preventing the urge to urinate frequently. The US Food and Drug Administration (FDA) approved Darifenacin for the treatment of overactive bladder on December 22, 2004.
Darifenacin may impair heat regulation. The patients are advised to use caution with activities in a hot environment leading to an increased core temperature of the body. The common side effects of the drug include blurred vision, headache, urinary tract infection, nausea, constipation, indigestion, and dry mouth.
How Does Darifenacin Work?
Darifenacin works by decreasing the contractions of an overactive bladder. It is an anticholinergic drug that relaxes the bladder muscles and increases the bladder's capacity. It is used to treat the signs of overactive bladder disorders in adults, including sudden urges to use the restroom immediately and for urgent urination.
Dosage:
-
The starting recommended dosage of Darifenacin is 7.5 mg once daily. Depending on each patient's response, the dose may be increased to 15 mg once daily.
-
Darifenacin should be taken with water once per day. It may be taken with or without food and should be completely swallowed without being chewed, crushed, or divided.
Warnings:
Urinary Retention: Darifenacin should only be used with caution in individuals with clinically considerable bladder outflow obstruction due to the possibility of urine retention.
Reduced Gastrointestinal Motility: Due to the possibility of stomach retention, Darifenacin should be administered cautiously to patients with obstructive gastrointestinal disorders. Darifenacin should be used cautiously in patients with severe constipation, ulcerative colitis, and irritable bowel syndrome since it, like other anticholinergic medications, may reduce gastrointestinal motility.
Angioedema: The use of Darifenacin has been reported to show symptoms of angioedema (swelling of the face, lips, tongue, or larynx) after the initial dose. Angioedema linked to swelling of the upper airways may be fatal. The drug will be stopped immediately if the patient's tongue, larynx, or hypopharynx is involved.
Controlled Narrow-Angle Glaucoma: Darifenacin should be used cautiously in patients with narrow-angle glaucoma as it may worsen the disease state.
Central Nervous System: Due to the anticholinergic action of Darifenacin, the administration of the drug can result in the development of the central nervous system (brain and spinal cord) side effects such as headache, disorientation, hallucinations, and sleepiness, particularly while starting the treatment or increasing the dose. Hence, patients should be observed for any such effects. They are also advised not to handle heavy machinery or a vehicle. If these side effects persist even after reducing the dose of the medication, discontinuation of the drug may be considered.
Liver Disease: Darifenacin is not advised for patients with severe liver impairment. However, in case of mild impairment, the physician adjusted the dose, which should not exceed 7.5 mg.
For Patients:
What Is Urinary Incontinence?
Urinary incontinence is the involuntary passage of urine. It is a common condition that affects women and men of all ages. Many factors, such as childbirth, aging, pregnancy, obesity, constipation or diarrhea, chronic coughing or sneezing, prostate surgery or prostate cancer treatment, diabetes, and other medical conditions, can cause urinary incontinence.
The most common symptom of urinary incontinence is an urgent need to urinate, followed by urine leakage. Other symptoms include a feeling of not being able to empty the bladder completely and uncontrolled urination at night (nocturia). Incontinence can affect people in many ways, such as their social life (example., avoiding social events), their sexual life (example., avoiding sexual intercourse), their self-esteem and self-confidence (example., feeling embarrassed), and their work life (example., taking time off work).
Learn More About Darifenacin:
Before Starting Darifenacin:
When and Why to Take Darifenacin?
Darifenacin is a prescription drug. Only use the medication as per the doctor's order. In case of doubts or queries concerning drug use, contact the doctor or pharmacist immediately. Talk with the doctor or pharmacist if the drug has a stronger or weaker effect than being indicated.
How Effective Is Darifenacin?
Studies have shown that the frequency of incontinence episodes/week decreased significantly with Darifenacin use. After two years, more than 40 % of patients had reduced their weekly incontinence occurrences by greater than or equal to 90 %.
Things to Inform The Doctor Before Taking Darifenacin:
Let the doctor know if any of the following conditions are present:
-
Autonomic Neuropathy: This condition is characterized by damage to the nerves that control important activities like heart rate, blood pressure, and bowel function by communicating with the brain and internal organs, muscles, skin, and blood vessels.
-
Experience frequent heartburn and belching.
-
Have a weak urine stream and trouble passing the urine.
-
Experience extreme constipation (less than or equal to two bowel movements per week).
-
Suffer from any disorder of digestive motility.
-
Have an obstructive gastrointestinal disorder (any restriction of the flow of stomach intestinal contents)
-
Using medications such as oral bisphosphonates might result in or exacerbate inflammation of the food pipe (a class of medicinal products that prevent bone mass loss and treat osteoporosis).
-
Have narrow-angle glaucoma and are undergoing therapy for it.
-
Experience liver issues.
-
Have a renal disease or a urinary tract infection.
-
Have heart disease.
-
All the prescription and over-the-counter medications, vitamins, and herbal supplements are currently being taken.
Starting Darifenacin:
How Is Darifenacin Given?
-
Darifenacin should be swallowed along with a glass of water or juice.
-
It may be taken with or without food.
-
Avoid crushing, chewing, or dividing the tablet.
What Are the Side Effects of Darifenacin?
The common side effects of Darifenacin include
-
Dry mouth.
-
Constipation.
-
Headache.
-
Dry eyes.
-
Indigestion.
-
Runny nose.
-
Dry eyes.
-
Abdominal pain.
-
Shortness of breath.
-
Fatigue.
-
Swelling of hands and ankles.
-
Drowsiness.
-
Dizziness.
-
Cough.
-
Facial swelling.
-
Formation of mouth ulcers.
-
High blood pressure.
-
Diarrhea.
-
Blurred vision.
-
Flatulence
-
Taste disturbance.
-
Urinary tract disorder or infection.
-
Vaginal discharge.
-
Mood changes.
-
Hallucinations.
Dietary Alterations:
Food or beverages that may increase urination should be avoided as it worsens the condition. Hence, food and beverages like coffee, pastries, colas, etc., containing caffeine may be limited to avoid the frequent urge to avoid. There are no other specific dietary alterations for the drug. However, if present with other disease conditions, follow the dietary restrictions as advised by the doctor or dietician.
What Should Be Done if a Dose Is Missed?
If a dose is forgotten, take it as soon as the patient remembers and continue with the regular dosing schedule. If the next dose is approaching, skip the prior one and carry on as planned. To make up for missing doses, avoid taking two doses at once.
What Should Be Done to Treat Darifenacin Overdose?
Do not take the tablet in excess of the recommended dosage. Take only the number of tablets listed on the pharmacy label by the doctor. If an overdose occurs, notify the doctor right away or go to the nearest accident and emergency facility.
How to Store Darifenacin?
-
Store Darifenacin at room temperature between 15 to 30 degrees Celsius.
-
Keep the medication away from children and pets.
-
The disposal of medications in wastewater or household waste is not recommended. Check how to dispose of expired medicines, according to a pharmacist. These actions will aid in keeping the environment safe.
Avoid Self-Medication:
Avoid using this medication if the patient does not have a prescription and does not take it on someone else's recommendation. Some people may even react worse to drugs than others, depending on how they are used. When seeing a doctor, keep a list of all the prescription and over-the-counter medications you are taking, and only take the drug as directed by a trained healthcare practitioner.
For Doctors
Indication:
Darifenacin is indicated for patients with overactive bladder.
Dosing:
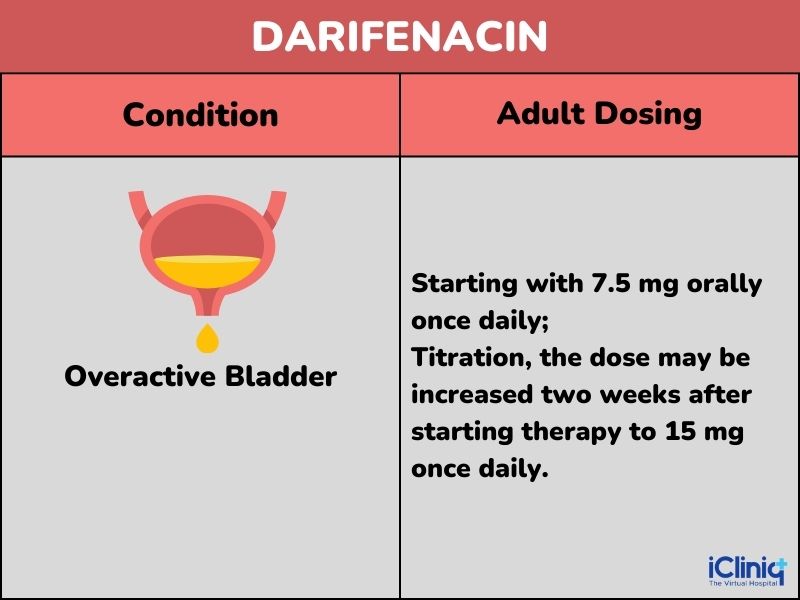
Dosing Considerations:
Renal Impairment: No dosage adjustment is required.
Mild Hepatic Impairment:
-
Child-Pugh A: No dosage adjustment is required.
-
Child-Pugh B: Do not exceed 7.5 mg orally daily.
-
Child-Pugh C: Use is not recommended.
Geriatric: No dose adjustment required.
Concurrent Use: Restrict the dose to a maximum of 7.5 mg in patients with concomitant use with potent CYP3A4 inhibitors like Ketoconazole, Itraconazole, Ritonavir, Nelfinavir, Clarithromycin, and Nefazodone.
Pharmacology:
Mechanism of Action:
Darifenacin Hydrobromide is a competitive muscarinic receptor antagonist with the greatest affinity for the M(3) receptor. Binding to the M(3) receptor facilitates cholinergic functions involving contraction of the human bladder and gastrointestinal smooth muscle, salivation, and iris sphincter function.
Darifenacin has a higher affinity for the M3 receptor than for the other known muscarinic receptors, according to in vitro investigations employing human recombinant muscarinic receptor subtypes. Muscarinic receptors are crucial for several key cholinergically mediated processes, including the stimulation of salivary production and contraction of the smooth muscle of the urine bladder. Drug side effects like constipation, dry mouth, and abnormal vision may be mediated by changes to M3 receptors in these organs.
Pharmacodynamics:
Increased bladder capacity was shown in three cystometric tests done on patients with involuntary detrusor contractions by an increased volume threshold for unstable contractions and a decreased frequency of unstable detrusor contractions after Darifenacin medication.
Electrophysiology:
In a multiple-dose, double-blind, randomized, placebo- and active-controlled (Moxifloxacin 400 mg) parallel-arm design study, 179 healthy adults (44 percent men, 56 percent women) between the ages of 18 and 65 were assessed for the effects of a six-day treatment with 15 mg and 75 mg of Darifenacin on the QT/QTc interval. There were 82 percent of extensive metabolizers and 18 percent of poor metabolizers (PMs) among the subjects (EMs). Predosing and steady-state QT intervals were measured during a 24-hour period. Because it produces exposure similar to that seen in CYP2D6 poor metabolizers given the maximum advised dose (15 mg) of darifenacin in the context of a powerful CYP3A4 inhibitor, the 75 mg Darifenacin dose was chosen.
At the doses studied, Darifenacin did not result in QT/QTc interval prolongation at any time during the steady state. In contrast, Moxifloxacin treatment resulted in a mean increase from baseline QTcF of about 7.0 msec when compared to placebo. In this study, Darifenacin 15 mg and 75 mg doses demonstrated a mean heart rate change of 3.1 and 1.3 bpm, respectively, when compared to the placebo.
Absorption:
The peak plasma levels of Darifenacin were attained seven hours after several doses of Darifenacin tablets were taken by healthy volunteers, and steady-state plasma levels were reached by the sixth day of therapy.
Distribution:
About 98 per cent of Darifenacin is bound to plasma proteins (primarily to alpha-1-acid-glycoprotein). The estimated steady-state volume of distribution (Vss) of the drug is 163 L.
Metabolism:
After an oral dose, the liver substantially metabolizes Darifenacin.
The cytochrome P450 enzymes CYP2D6 and CYP3A4 mediate metabolism. The three primary metabolic pathways are
-
Dihydro benzofuran ring's monohydroxylation.
-
Opening of the dihydro benzofuran ring.
-
The nitrogen of the pyrrolidine is N-dealkylated.
The primary circulating metabolites are the early byproducts of the hydroxylation and N-dealkylation processes. However, it is doubtful that they will significantly impact the therapeutic effect of Darifenacin as a whole.
Excretion:
Healthy participants were given an oral dose of a 14C-Darifenacin solution, and afterward, 40 per cent of the radioactivity was recovered in the feces and 60 per cent in the urine. Only three per cent of the excreted dosage was darifenacin which had not been changed. Darifenacin clearance is anticipated to be 40 L/h for EMs and 32 L/h for PMs. Following chronic dosage, Darifenacin has an elimination half-life of roughly 13 to 19 hours.
Active Ingredient: The active ingredient in the Darifenacin tablet is Darifenacin hydrobromide.
Inactive Ingredient: The other ingredients are
-
Anhydrous calcium hydrogen phosphate.
-
Magnesium stearate.
-
Titanium dioxide.
-
Hypromellose.
-
Polyethylene glycol.
-
Talc.
-
Red iron oxide.
-
Yellow iron oxide.
Toxicity:
Non-clinical Toxicology:
Darifenacin has not been related to drug-related carcinogenicity and genotoxicity. Animal studies indicated no impairment in fertility in male and female mice.
Clinical Toxicology:
Mild to Moderate Toxicity:
It is an extension of the pharmacologic effects due to extensive antagonism of the central and peripheral muscarinic acetylcholine receptors. The epidemiology of poisoning is frequent but rarely fatal. Toxicity can manifest itself orally, parenterally, or topically. Ophthalmic preparation has occasionally resulted in systemic anticholinergic effects, mainly in young infants.
Management: Characteristic symptoms include somnolence, tachycardia, anticholinergic effects of mydriasis, flushing, fever, dry mouth, and reduced bowel noises. After an overdose, mild hypertension, nausea, and vomiting are also typical. With moderate poisoning, agitation, disorientation, and hallucinations may appear.
Severe Toxicity:
If the patient shows up soon after ingesting the drug, administer activated charcoal; sedate patients with benzodiazepines for agitation and delirium. Since tachycardia is typically low and readily tolerated, no special care is needed. Physostigmine can be used to diagnose; however, it should only be administered in a setting where thorough monitoring and resuscitation are available. It should also not be administered if the history or ECG (QRS widening) suggests tricyclic antidepressant use.
Management: Early orotracheal intubation is necessary to protect the airway. If the patient arrives quickly after a heavy intake, gastric lavage may be beneficial; activated charcoal should be given. Only patients who can defend their airways or who are intubated should undergo GI (gastrointestinal) decontamination. Large doses of benzodiazepines are often needed to sedate severe delirium. Barbiturates, Propofol, or benzodiazepines may need to be used aggressively if there are seizures (which could escalate to status epilepticus). Keeping an eye on body temperature and treating hyperthermia with severe benzodiazepine sedation to calm agitation and external cooling. In the case of anticholinergic ileus, delayed absorption may cause clinical symptoms to last longer.
Warnings and Precautions:
-
Beers Criteria: Avoid using it in elderly patients who are already experiencing delirium or who are at high risk of developing it because it may cause or exacerbate delirium, in patients who have dementia or cognitive impairment because of negative CNS (central nervous system) effects, and in men who have symptoms of the lower urinary tract or benign prostatic hyperplasia because it may cause decreased urinary flow and urinary retention.
-
Confusion, slumber, headaches, and hallucinations are possible anticholinergic CNS side effects; monitoring is advised; It could be essential to reduce or stop the dosage. There have been reports of angioedema of the face, tongue, lips, and/or throat; if this happens, stop using the medication immediately.
-
Bladder outflow blockage, considerable; increased risk of urinary retention; severe constipation; possible decrease in gastrointestinal motility; increased risk of gastric retention; mild gastrointestinal obstructive disorders; necessary dose modifications; people with severe hepatic impairment should not use.
-
Myasthenia gravis; managed narrow-angle glaucoma; impaired gastrointestinal motility.
-
Ulcerative colitis; possible reduction in gastrointestinal motility.
Contraindications:
Darifenacin is contraindicated under the following conditions:
-
In people at risk or with pre-existing gastric retention.
-
In people at risk or with pre-existing or uncontrolled narrow-angle glaucoma, uncontrolled.
-
In people at risk or with pre-existing urinary retention.
-
Along with drugs like Levoketoconazole, Potassium Citrate, and Potassium.
Clinical Studies:
In three randomized, fixed-dose, placebo-controlled, multicenter, double-blind, 12-week studies (Studies 1, 2, and 3), as well as one randomized, double-blind, placebo-controlled, multicenter, dose-titration study, Darifenacin extended-release tablets were assessed for the treatment of patients with overactive bladder with symptoms of urinary incontinence, urgency, urinary incontinence, and increased urinary frequency.
In order to be eligible for the research, patients had to have at least eight micturitions per day, at least one incident of urinary urgency, and at least five episodes of urinary incontinence per week while experiencing symptoms of overactive bladder for at least six months.
Most patients (94 per cent) and women (84 per cent) were white; their average age was 58 years, ranging from 19 to 93 years. 33 percent of patients were over 65 years old. These traits were evenly distributed amongst treatment groups. The study population included both new patients (60 per cent) who had not previously received medication for overactive bladder and those who had (40 percent).
Results:
Within the first two weeks of treatment, patients receiving Darifenacin 7.5 mg and 15 mg once daily saw a decrease in urge incontinence incidents per week compared to placebo. Additionally, these outcomes persisted over the course of the 12-week therapy period.
Drug Interactions:
-
The cytochrome P450 enzymes CYP2D6 and CYP3A4 play a key role in the metabolism of Darifenacin. As a result, CYP3A4 inducers or inhibitors of either of these enzymes may change the pharmacokinetics of the drug.
The drug-drug interactions of Darifenacin are as follows:
-
Amifampridine.
-
Belzutifan.
-
Bupropion.
-
Ceritinib.
-
Clozapine.
-
Conivaptan.
-
Desipramine.
-
Donepezil.
-
Duvelisib.
-
Fedratinib.
-
Fexinidazole.
-
Flecainide.
-
Fosnetupitant.
-
Glucagon.
-
Glycopyrrolate.
-
Glycopyrronium Tosylate.
-
Idelalisib.
-
Imipramine.
-
Itraconazole.
-
Ivosidenib.
-
Larotrectinib.
-
Lefamulin.
-
Lorlatinib.
-
Lumacaftor.
-
Methacholine.
-
Netupitant.
-
Pacritinib.
-
Quetiapine.
-
Revefenacin.
-
Scopolamine.
-
Secretin Human.
-
Thioridazine.
-
Tiotropium.
-
Ketoconazole.
Other Specifications:
Darifenacin in Pregnant and Lactating Women:
-
Darifenacin is classified under pregnancy category C. It has not been studied in pregnant women. Darifenacin should only be used during pregnancy if the benefit to the mother outweighs the potential risk to the fetus, as research on animal reproduction is not necessarily indicative of human response.
-
It is unknown if Darifenacin is passed on to breast milk. Hence, caution is required while administering the drug to breastfeeding mothers.
Darifenacin in Pediatric Patients:
The safety and efficacy of Darifenacin are not yet established in children.
Darifenacin in Geriatric Patients:
No specific dosing recommendations are required for geriatric patients.
Darifenacin in Patients With Renal Impairment:
No dose adjustment is recommended for patients with renal impairment.
Darifenacin in Patients With Hepatic Impairment:
No dose change is recommended for patients with mild to moderate hepatic impairment. Darifenacin is not advised for use in individuals with severe hepatic impairment (Child-Pugh C), as these subjects have not been examined.












