Introduction:
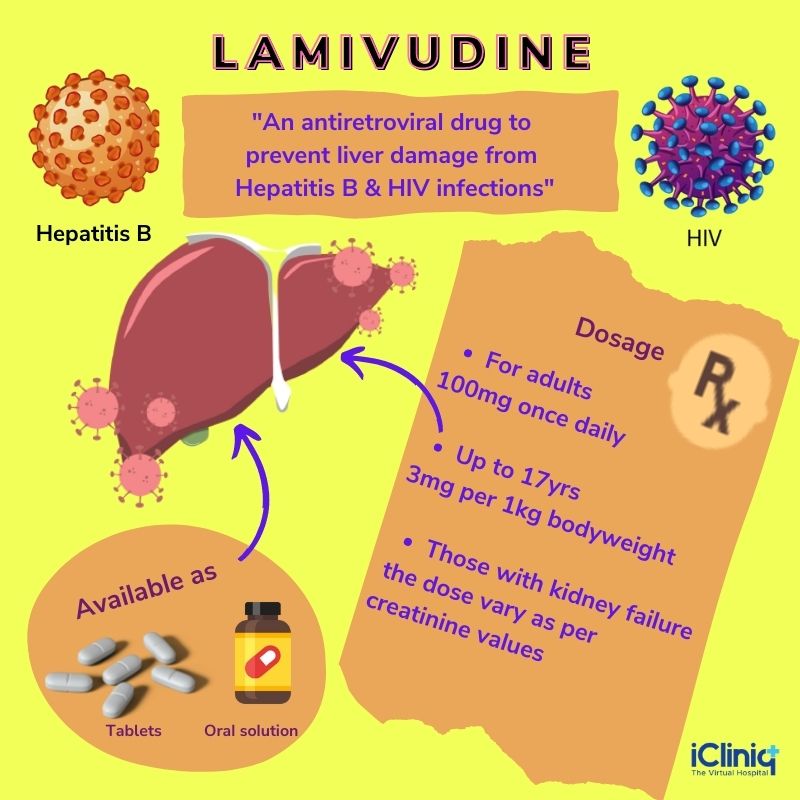
Lamivudine-HBV (hepatitis B virus) is an antiretroviral medication and a synthetic nucleoside analog used to treat chronic hepatitis B virus infection associated with hepatitis B virus replication and inflammation of the liver. The drug works by reducing the amount of HIV (human immunodeficiency virus) and hepatitis B in the blood. Although Lamivudine does not treat or cure hepatitis B permanently, it retards the disease progression and reduces the chances of liver damage. The main advantage of Lamivudine is that it is used not only for hepatitis B but also in combination with other antiretroviral medications for managing HIV. Normally, HIV and HBV spread and replicate in the body with the help of the enzyme known as the reverse transcriptase. However, Lamivudine works by blocking this enzyme preventing its further replication.
How and When Was the Drug Developed and Approved?
-
Lamivudine was developed and invented by Bernard Belleau and Nghe Nguyen-Ga at the McGill University and Montreal-based IAF Biochem International Inc. laboratories in 1989.
-
The drug samples were sent to Yung-Chi Cheng of Yale university for their evaluation of toxicity. It was discovered that when Lamivudine is used in combination with other antiviral drugs, it inhibits the reverse transcriptase enzyme and shows an increased efficacy.
-
Studies report that Lamivudine is less toxic to the mitochondrial DNA (deoxyribonucleic acid) than the other retroviral drugs.
-
Finally, Lamivudine was approved by the Food and Drug Administration (FDA) on 17th November 1995 and again in 2002 to be used as a once-in-a-daily dose medication.
-
Currently, the drug is licensed to and manufactured by GlaxoSmithKline.
General Information About Lamivudine:
How Does Lamivudine Work to Treat Hepatitis B?
Hepatitis B occurs due to viral infection and is characterized by inflammation of the liver. It belongs to a class of drugs known as nucleoside reverse transcriptase inhibitors. These drugs work and treat the condition in a similar manner. This drug is an analog of cytidine and is considered effective as it inhibits the reverse transcriptase of HIV-1 and -2 and the hepatitis B virus. Lamivudine undergoes phosphorylation to form active metabolites that compete to get incorporated into the viral DNA. Lamivudine blocks the secretion of the reverse enzyme transcriptase, which is required by the HIV and HBV viruses for replication or making copies. As a result, delayed growth of the HBV virus is commonly observed. Lamivudine is usually taken orally and is known to delay the progression of the disease.
What Has Been Known Regarding the Safety and Effectiveness of Lamivudine?
It has been observed that hepatitis B progresses to liver failure in more than 1 % of the patients. As a result, these patients might have to undergo a liver transplantation procedure. However, during the clinical trials, when patients with chronic and exacerbating hepatitis B were treated with Lamivudine to prevent the recurrence of hepatitis B after liver transplantation, it was noted that the patients recovered fully. The patients had their normalized prothrombin time restored to its normal level within a week. The best part about the drug is that none of the patients reported an adverse reaction. Therefore, the data obtained from the clinical trial suggests that Lamivudine is safe and effective in patients with severe or fulminant hepatitis B. The patients recovered rapidly when the drug was administered at the right time.
What Are the Uses of Lamivudine?
Lamivudine is an antiviral drug used in combination with other drugs to treat HIV-1 and HIV-2. It is also indicated for managing long-standing hepatitis B virus infection associated with the evidence of liver inflammation and viral replication. It can be used at a lower dose than the one indicated for HIV to manage hepatitis B. The drug is beneficial for hepatitis B because it improves seroconversion and the histological staging of the liver. Although the long-term use of Lamivudine might lead to the emergence of a resistant hepatitis B virus (YMDD) mutant, it is widely used as the drug is well tolerated.
Dosage and Administration:
Counseling and Testing for HIV:
All patients must be tested and counseled for HIV before initiating and during the treatment with Lamivudine for hepatitis B virus. This is because there is always a risk of the emergence of resistant HIV-1 and a limitation of treatment options if the drug is prescribed to a patient with chronic hepatitis B and acquires an unrecognized HIV-1 infection during the treatment.
Recommended Dosage of Lamivudine for Adult Patients:
The recommended dosage of Lamivudine for adult patients with chronic hepatitis B is 100 mg once daily.
Recommended Dosage of the Drug for Pediatric Patients:
The recommended dosage of Lamivudine for children between two to 17 years of age is 3 mg per kg once daily. The maximum dose of the drug is 100 mg. Patients who need less than 100 mg of the drug must be given an oral solution formulation as they might be unable to swallow the tablets.
Patients Suffering From Renal or Kidney Impairment:
The dosage of Lamivudine for patients with renal impairment depends upon their creatinine clearance. The dosage as per the creatinine clearance has been described in the table below:
No additional dosage modifications are required for patients taking Lamivudine after fours of hemodialysis or peritoneal dialysis. However, no data is available regarding the dosage of Lamivudine in pediatric patients with renal impairment.
General Information About Hepatitis B:
Hepatitis B is an infection of the liver mainly caused by the hepatitis B virus. Sometimes, the patient might have a chronic hepatitis B infection, which might last for more than six months. The condition might become life-threatening as the virus spreads through blood, semen, and other body fluids. Hepatitis B increases the risk of liver failure, cirrhosis, and liver cancer. It has been noticed that adults often recover from hepatitis B, even if the symptoms are severe. However, infants and young children might remain infected for prolonged periods. A vaccine can help prevent the condition but does not permanently cure it. However, the disease can be prevented if the patient takes the required precautions.
Signs and Symptoms of Hepatitis B:
The signs and symptoms of hepatitis B vary from one individual to the other. The symptoms are usually noted one to four months after the viral infection.
The symptoms of hepatitis B are listed below:
-
Abdominal pain.
-
Dark urine.
-
Pain in the joints.
-
Loss of appetite.
-
Nausea.
-
Weakness.
-
Fatigue.
-
The skin and the whites of the eyes become yellow.
For Patients:
Lamivudine is a prescription medication used to manage chronic or long-standing hepatitis B virus when the disease progresses severely, and there is liver inflammation or swelling.
It has not been known whether Lamivudine is effective in:
-
Patients with long-standing HIV and severely damaged liver (decompensated liver disease).
-
Patients with HIV-1, hepatitis C, and hepatitis D (delta) virus.
-
Patients who have undergone the liver transplant procedure.
-
Children who are less than two years of age and have chronic hepatitis B.
What Is the Most Important Information the Patient Should Know About Lamivudine?
The patient must know the following information related to Lamivudine:
-
Lamivudine can worsen liver disease and hepatitis B infection after the patient stops taking it. Sometimes, the condition becomes severe, resulting in the death of the person. The patients planning to take Lamivudine must remain in constant touch with their doctors and undergo liver function tests and blood tests.
-
The patients infected with HIV-1 must be counseled before initiating the treatment with Lamivudine, as the virus might become resistant to the drug. As a result, it becomes difficult to treat the underlying HIV infection.
-
There are chances of mutations or changes in the hepatitis B virus during the treatment with Lamivudine. As a result, the patient might have an incurable infection and a life-threatening condition.
What Should the Patient Inform the Doctor Before Taking Lamivudine?
Before taking Lamivudine, the patient must inform the doctor if he or she has:
-
HIV-1 infection.
-
Kidney problems.
-
Diabetes because every 20 mL or 100 mg dose of Lamivudine oral solution contains 4 grams of sucrose.
-
Conceived or planning to conceive. However, nothing has been known about the effects of Lamivudine on the unborn baby. Females who are planning to get pregnant and take Lamivudine can become a part of the pregnancy registry, where they need to submit all the information regarding their pregnancy.
-
Breastfed or planning to feed the baby. This is because Lamivudine can pass from the mother’s milk into the fetus and harm them. The doctor will decide whether the patient should take Lamivudine or not.
-
Taken or is taking any prescription, over-the-counter drugs, and other medications, including herbal supplements and vitamins. The patient must keep a list of all the medications and show it to his doctor or pharmacist.
-
The patient can also consult the doctor to know about the medications that interact with Lamivudine.
-
Avoid taking a new medication without consulting the doctor because only the doctor can guide whether Lamivudine can be taken with other medications or not.
How Should the Patient Take Lamivudine?
-
The patient must take Lamivudine exactly as mentioned by the doctor.
-
If the patient misses the dose of Lamivudine, he must take it immediately. Avoid taking two doses simultaneously. Do not take more than the dose prescribed by the health care provider.
-
The patient must be continuously monitored by the doctor during his therapy with Lamivudine.
-
The doctor will prescribe the medication based on the weight of children between 2 to 17 years of age.
-
The patient must inform the doctor if he or his child has difficulty swallowing the tablets. Lamivudine is also available as a liquid or oral solution.
-
If the patient consumes Lamivudine excessively, he must call the doctor or visit the nearest emergency room immediately.
What Are Some of the Possible Side Effects of Lamivudine?
Lamivudine can cause the following serious side effects:
1. It can lead to the accumulation of lactic acid in the blood, resulting in a condition known as lactic acidosis. Although this condition is noticed in only some patients, it is a serious and life-threatening disorder. The signs of lactic acidosis are:
- Weakness or tiredness.
- Abnormal muscle pain.
- Respiratory difficulties.
- Stomach pain.
- Nausea.
- Vomiting.
- The patient feels cold in his arms and legs.
- Dizziness or lightheadedness.
- Fast or irregular heartbeat.
2. The patients taking Lamivudine or other medications might have severe problems in the liver. Sometimes, liver problems become severe to the extent that they result in death. The liver might become enlarged, resulting in hepatomegaly and fatty liver (steatosis). The patient must consult the doctor if he gets the following signs and symptoms due to liver problems:
- The skin and the whites of the eyes turn yellow.
- Tea-colored urine.
- Light-colored stools.
- Loss of appetite for a few days.
- Nausea.
- Pain and tenderness on the right side of the abdomen.
3. The most common side effects of Lamivudine include infections in the ear, nose, and throat, resulting in diarrhea and sore throat.
Safety and Effective Use of Lamivudine:
The doctor might prescribe the medication for purposes other than those enlisted in the patient information leaflet. However, the patient must ensure that he avoids the use of the drug for the condition for which it was not prescribed. In addition, the patient must refrain from administering Lamivudine to others even if they have similar symptoms, as it might harm them. Finally, the patient can consult the doctor for more information regarding the drug.
For Doctors:
Description:
Lamivudine is a synthetic nucleoside analog with potent activity against the hepatitis B virus. The chemical name of the drug is (2R,cis)-4-amino-1-(2-hydroxymethyl-1,3-oxathiolan-5-yl)-(1H)-pyrimidine-2-one. Structurally, Lamivudine is an enantiomer of the dideoxy analog of cytidine and can also be referred to as (-)2,3-dideoxy, 3-thiacytidine. The molecular formula of Lamivudine is C8H11N3O3, and the molecular weight of the drug is 229.3 g per mol. It is a white or off-white crystalline solid with a solubility of approximately 70 mg per mL in water at 20 degrees Celsius. Lamivudine tablets must be administered orally only.
Indications and Usage:
Lamivudine has been indicated for managing hepatitis B virus infection associated with the evidence of hepatitis B viral replication and acute liver inflammation.
Therefore, the doctor must consider the following points while initiating the treatment with Lamivudine:
-
Patients taking Lamivudine have demonstrated high rates of resistance after the initiation of the treatment, so the treatment with Lamivudine must only be considered when an alternative antiviral drug with a high genetic barrier to resistance is unavailable.
-
The use of Lamivudine has not been evaluated in patients who have HIV, hepatitis C virus, and the hepatitis delta virus.
-
Lamivudine has also not been evaluated in patients who received a liver transplant or have chronic hepatitis B virus infection and decompensated liver disease.
Chemical Taxonomy:
Clinical Pharmacology:
Microbiology and Mechanism of Action:
Lamivudine is an analog of a synthetic nucleoside. The drug is mainly phosphorylated to its active metabolite 5’-triphosphate, Lamivudine triphosphate (3 TC-TP) within the cells. The main mechanism of action of 3 TC-TP is the inhibition of DNA and RNA-dependent polymerase activities of the hepatitis B virus reverse transcriptase via DNA chain termination procedure.
Antiviral Activity:
The activity of Lamivudine against the hepatitis B virus was assessed during the cell culture in the following cells:
-
HBV (hepatitis B) DNA-transfected cells.
-
2.2.15 cells.
-
HB611 cells.
-
Infected human primary hepatocytes.
Variations were noted in the EC50 values from 0.01 microns to 5.6 microns depending upon the following factors:
-
Duration of exposure of the cells to Lamivudine.
-
The cell model system.
-
The protocol used.
Resistance:
Lamivudine-resistant isolates were discovered in people with a virologic breakthrough. These isolates have been known to develop rtM204V/I substitutions in the catalytic domain of the viral reverse transcriptase. rtM204V/I substitutions might be accompanied by other substitutions, which usually enhance the levels of Lamivudine resistance. They can also work as compensatory substitutions to improve replication efficiency. The other substitutions that were noted in the Lamivudine-resistant isolates are listed below:
-
rtH55R.
-
rtL80I/V.
-
rtV173M.
-
rtA181T/V.
-
rtT184S.
-
rtF219Y.
-
rtL229F/M/V/W.
-
rtQ267H.
Cross-Resistance:
Hepatitis B virus containing Lamivudine resistance-associated substitutions have susceptibility to Adefovir Dipivoxil but reduced susceptibility to Entecavir and Telbivudine.
Composition of Lamivudine:
1. Active Ingredients - Lamivudine.
2. Inactive ingredients:
- Lamivudine Tablets - Hypromellose, Microcrystalline cellulose, Red iron oxide, Sodium starch Glycolate, magnesium stearate, polysorbate 80, Macrogol 400, titanium dioxide, and yellow iron oxide.
- Lamivudine Oral Solution - Artificial strawberry and banana flavors, anhydrous citric acid, Propylene glycol, Methylparaben, Sodium citrate dihydrate, Sucrose 200 mg per mL, and Propylparaben.
Pharmacokinetics:
Pharmacokinetics in Adults:
Single and multiple doses of Lamivudine 5 mg to 600 mg were administered to the patients to evaluate its pharmacokinetic properties.
Absorption and Bioavailability:
When the patients were given a single oral dose of 100 mg, the peak serum concentration in hepatitis B infected patients, and healthy was noted to be 1.28 +/- 0.56 mcg per mL and 1.05 +/- 0.32 mcg per mL, which occurred after two hours of administration of the drug. Though the Lamivudine solution demonstrated a slightly higher peak serum concentration, no significant differences in the systemic exposure of the drug were noted.
Effects of Food on Oral Absorption:
Lamivudine tablets and oral solutions can be taken with or without food. During the clinical trial, no clinically significant differences were noted when Lamivudine was administered to patients with and without food.
Distribution:
The apparent drug distribution volume after intravenous administration was 1.3 +/- 0.4 L per Kg. This value suggests that Lamivudine gets distributed to the extravascular spaces also. Hence, the drug distribution volume was dose-dependent and not correlated with body weight. The binding of Lamivudine to human plasma protein is less than 36 %. In vitro studies have demonstrated that the amount of Lamivudine associated with the erythrocytes ranged from 53 to 57 % over the concentration range of 0.1 to 100 mcg per mL.
Metabolism:
During the research, it was discovered that the only recognized metabolite of Lamivudine is a trans-sulfoxide metabolite. However, nothing has been known about the serum concentration of this metabolite. In addition, Lamivudine is not metabolized by the cytochrome P450 enzymes.
Elimination:
The majority of the drug is eliminated unchanged in the urine by organic cationic secretion. Therefore, the drug's oral clearance and elimination half-life are independent of the dose and body weight.
Non-Clinical Toxicology:
Carcinogenesis: No carcinogenic effects of Lamivudine were noted during the animal studies. However, some effects were observed in humans at the therapeutic dose.
Mutagenesis: Some pieces of evidence of mutagenicity were observed in animal studies after Lamivudine was administered.
Impairment of fertility: Lamivudine did not have any impact on male or female fertility.
Dosage Forms and Strength of Lamivudine:
Lamivudine Tablets - They are available as 100 mg butterscotch-colored, film-coated, biconvex, and capsule-shaped tablets imprinted with GX CG5 on one side.
Lamivudine Oral Solution - The solution is available as a clear, colorless to pale yellow, strawberry, and banana-flavored liquid, having 5 mg of Lamivudine per 1 mL.
Contraindications: Lamivudine is contraindicated in patients with a previous history of hypersensitivity reactions.
Warnings and Precautions for Lamivudine:
-
Hepatitis Exacerbation - During the clinical and laboratory research, exacerbations of hepatitis were observed after the discontinuation of Lamivudine. These exacerbations were detected by serum ALT elevations and the reemergence of hepatitis B virus DNA after the treatment was stopped. Though the events of exacerbations were self-limiting, fatalities were reported in some cases. Patients who have stopped taking Lamivudine must be closely monitored and kept on follow-ups for several months.
-
Risk of HIV-1 Resistance - Lamivudine is prescribed at a lower dose in patients with hepatitis B than in HIV. Therefore, the patients coinfected with HIV and hepatitis B must not be administered Lamivudine because the risk of emergence of HIV-1 resistance is likely to increase. The risk increases mainly due to the inappropriate use of monotherapy and sub-therapeutic doses for HIV-1 treatment. It is important to give HIV counseling and testing to the patients before beginning with and during the treatment with Lamivudine
-
Resistance-Associated Hepatitis B Substitutions - During the controlled clinical trials, YMDD mutant hepatitis B was observed in patients taking Lamivudine. The patients demonstrating YMDD mutants treated with Lamivudine displayed a diminished treatment response compared to the ones with evidence of YMDD substitutions. Severe progression of hepatitis B and incidences of death were also reported in patients with YMDD-mutant hepatitis B. Therefore, the doctor must suggest an alternative treatment option for these patients to lower the risk of death, hepatitis B progression, and seroconversion.
-
Lactoacidosis and Severe Hepatomegaly - Lactoacidosis and severe hepatomegaly accompanied by steatosis have been reported in patients on Lamivudine therapy. A majority of such fatal outcomes have been observed in females. Obesity is also one of the risk factors for lactoacidois and steatosis in patients treated with antiretroviral nucleosides. So, the treatment with Lamivudine must be suspended if the patient exhibits symptoms of lactoacidosis and severe hepatomegaly, even in the absence of transaminase elevations.
What Are the Adverse Reactions of Lamivudine?
The following adverse reactions were consistently seen in patients during and after the clinical trial:
-
Ear, nose, and throat infections.
-
Sore throat.
-
Diarrhea.
Post-Marketing Experience - The following side effects were observed in a small group of the population after the drug was launched in the market:
-
Anemia, including pure red cell aplasia.
-
Lymphadenopathy.
-
Splenomegaly.
-
Thrombocytopenia.
-
Stomatitis.
-
Hyperglycemia.
-
Weakness.
-
Lactic acidosis.
-
Steatosis.
-
Post-treatment exacerbations of hepatitis.
-
Anaphylaxis.
-
Urticaria.
-
Cramps.
-
Rhabdomyolysis.
-
Paresthesia.
-
Peripheral neuropathy.
-
Wheezing.
-
Abnormal breath sounds.
-
Alopecia.
-
Pruritus.
-
Rashes.
Drug Interactions Studies:
-
Effect of Lamivudine on Other Agents - It has been noted that Lamivudine is least likely to affect the other drugs at therapeutic doses. The drugs which are the transporters of the following do not exhibit any change in their pharmacokinetics after Lamivudine is administered:
-
Organic anion transporter polypeptide 1B1/3 (OATP1B1/3).
-
Breast cancer resistance protein (BCRP).
-
P-glycoprotein (P-gp), multidrug and toxin extrusion protein 1 (MATE1), and organic cation transporter 1 (OCT1), OCT2, or OCT3.
-
-
Effects of Other Drugs on Lamivudine - Trimethoprim increased the plasma concentrations of Lamivudine during the in vitro studies. However, this drug interaction is not clinically significant and requires no dose adjustment.
-
Interferon Alfa - There were no significant alterations in the pharmacokinetics of Lamivduine when Interferon alfa was administered.
-
Ribavirin - In vitro studies indicate that Ribavirin decreases the phosphorylation of Lamivudine, but no pharmacokinetic or pharmacodynamic interactions were observed.
-
Sorbitol - Lamivudine and Sorbitol were administered simultaneously in patients during the cross-over clinical trial. A dose dependent reduction in the Cmax was reported in patients taking Lamivudine.
-
Trimethoprim or Sulfomethoxazole - Trimethorpim decreased the renal and oral clearance of Lamivudine when they were coadministered. However, Lamivudine did not alter the pharmacokinetics of Trimethoprim or Sulfamethoxazole.
Important Instructions for Drug Administration:
-
Lamivudine tablets and oral solution can be taken with or without food.
-
The oral solution and tablets can be used interchangeably.
-
The oral solution is recommended for doses less than 100 mg.
Assessment of the Patients During the Treatment:
The patients taking Lamivudine must be constantly monitored by an experienced physician. The following observations must be taken into account while monitoring the patient:
-
Return of elevated ALT levels.
-
Increase in the levels of hepatitis B virus DNA after an initial decline below the assay limit.
-
Progression of the signs and symptoms of liver disease.
-
Worsening of the hepatic necroinflammatory findings.
How Is the Drug Supplied and Handled?
Lamivudine tablets are butterscotch-colored and film-coated. They are packed in bottles containing 60 tablets with a closure containing information about child resistance. The tablets must be stored at 25 degrees Celsius. Lamivudine oral solution is clear and colorless to pale yellow. It is packaged in plastic bottles of 240 mL with a child-resistant closure. The oral solution does not need to be reconstituted and can be stored at temperatures between 20 to 25 degrees Celsius.
Use in a Specific Population:
Pregnancy: Not much information is available regarding the effect of Lamivudine on pregnant females. However, embryolethality was observed during the animal studies. In addition, there is a pregnancy registry that carefully evaluates the effects of Lamivudine on pregnant females. When administered to pregnant females, no differences were observed in the drug's pharmacokinetics. However, when the amniotic fluid specimens were collected, it was observed that Lamivudine crossed the placenta and ruptured the membranes.
Lactation: Studies report that Lamivudine is present in milk, but nothing has been known about the concentration of the drug in lactating females infected with hepatitis B. The lactating females infected with HIV had a higher concentration of Lamivudine than those infected with the hepatitis B virus. However, nothing has been known about the effects of the drug on the breastfed infant. Therefore, the health benefits of Lamivudine must be considered in lactating females before administering the drug.
Pediatric Use: Lamivudine can be safely administered to children between 2 to 17 years of age. However, nothing has been known about the safety and efficacy of the drug in patients below two years of age.
Geriatric Use: There is insufficient information available regarding the side effects of Lamivudine in the geriatric age group because only a few patients above 65 years were included in the trial. However, the drug must be carefully administered in these patients, and the risk-benefit ratio related to the hepatic and renal side effects must be outweighed.
Patients With Kidney Disorders: The dosage of Lamivudine must be reduced in people suffering from kidney disorders because the drug gets eliminated directly from the urine.
Patients With Damaged Liver: The dosage of Lamivudine need not be adjusted for patients suffering from liver damage.
Overdosage:
Nothing has been known related to the treatment of overdosage of Lamivudine. However, if an overdose occurs, the patient should be constantly monitored and must be given supportive treatment. Studies report that a negligible amount of the drug was removed by hemodialysis, continuous ambulatory dialysis, peritoneal dialysis, and automated peritoneal dialysis. However, nothing has been known about the efficacy of continuous hemodialysis in this regard.
Clinical Trial:
The safety and efficacy of Lamivudine 100 mg were evaluated in three controlled clinical trials in patients with compensated hepatitis B. The trial participants were more than 16 years of age and had a long-standing hepatitis B infection, and showed evidence of hepatitis B virus replication.
Trial 1: A randomized, double-blind trial of Lamivudine 100 mg once daily and placebo for 52 weeks, followed by a no-treatment period of 16 weeks.
Trial 2: A randomized, double-blind three-arm trial was done to compare Lamivudine 25 mg once daily versus Lamivudine 100 mg and placebo for 52 weeks.
Trial 3: A randomized, partially blind trial was done on 238 patients who had active and long-standing hepatitis B despite receiving treatment with interferon alfa. The trial was done to compare Lamivudine 100 mg once daily for 52 weeks, followed by Lamivudine 100 mg or a matching placebo once daily for 16 weeks.
Trial Results:
It was observed that most of the patients treated with Lamivudine showed a decrease in the hepatitis B virus DNA below the assay limit during the therapy. However, one-third of the patients demonstrated reemergence of assay detectable hepatitis B virus DNA during the treatment with Lamivudine.












